Parasitic infections are an uncommon but potentially important cause of morbidity and mortality in children undergoing solid organ transplantation (SOT). Although few data have been published relating to parasitic infections in children undergoing hematopoietic stem cell transplantation (HSCT) or being treated for cancer, the high degree of immunodeficiency relating to treatment with chemotherapy and ablation therapy early after HSCT transplant, as well as the potential presence of ongoing immunosuppression treatment to prevent or treat graft-versus-host disease (GVHD), also places these children at increased risk for serious disease if they are exposed to these parasitic pathogens. This chapter reviews several important parasitic pathogens and their impact on these immunosuppressed children.
Strongyloidiasis
Epidemiology and risk factors
There are more than 40 species within the genus of Strongyloides; however, the main species that infects humans is S. stercoralis . It is an intestinal nematode predominantly present in the subtropics and tropical areas as well as in the Appalachian area and southeastern United States. It is estimated that between 30 million and 100 million people worldwide are infected with Strongyloides spp. , S. rhabditiform larvae are excreted in stool of infected individuals and either develop into free-living adult worms or into the filariform larvae. During the free-living adult worm stage, they can produce fertilized eggs that can then develop into the rhabditiform larvae. , Transmission often occurs when the filariform larvae penetrate the skin of a person walking barefoot on soil in endemic areas. Larvae then travel to the intestines and mature into adult worms. It is the only nematode that can cause autoinfection once it completes the life cycle within a human host. , In autoinfection, adult female worm lay eggs within the intestinal mucosa that become rhabditiform larvae. Subsequently, these larvae develop into the filariform larvae and can penetrate the intestinal mucosa or perianal skin and migrate to the intestines via the lungs to restart the cycle.
Possible outcomes to an initial infection are eradication of the infection, autoinfection, and hyperinfection or disseminated disease, which is rare in immunocompetent hosts. Transmission from human to human is extremely rare. Transmission can occur as part of SOT seen as donor-derived infections, * which is most often associated with kidney transplantation (KTx) with an increased number of hyperinfections related to corticosteroid use. , , Infections in bone marrow transplant recipients have been rarely reported. Hyperinfection occurs during autoinfection, with an increase larval migration into the pulmonary system. In disseminated disease, larvae migrate within the venous system to reach other organs. In disseminated disease, there is an increased risk of enteric gram-negative bacteremia and meningitis. Data on Strongyloides in children being treated for cancer are not available.
The main epidemiologic risk factor for developing strongyloidiasis is living or visiting endemic areas, such as Central and South America, the Caribbean, Puerto Rico, Mexico, sub-Saharan Africa, Asia, India, and Oceania. The highest seropositivity in these areas can exceed 80% as opposed to only 3.8% in the United States, yet 6.7% of pretransplant evaluations reveal positive serology results in asymptomatic Hispanic transplant candidates.
Clinical manifestations
Most infections are asymptomatic, but strongyloidiasis can present with abdominal pain, diarrhea, bloating, anorexia, cough, sore throat, or rash in the immunocompetent patient. , Owing to the use of immunosuppression, including corticosteroids, SOT recipients can present with gastrointestinal symptoms, respiratory distress, sepsis-like picture, bacteremia, and/or meningitis. , The pathogenesis stems from autoinfection through intestinal mucosa allowing for bacterial seeding. In disseminated disease, end-organ dysfunction specific to the larval migration is seen. Eosinophilia can be present in up to 30% of patients with hyperinfection syndrome. , , Mortality associated with hyperinfection can range from 25% to 87%, with better outcomes depending on early detection. , , Patients have also developed acute respiratory distress syndrome as a complication of hyperinfection.
Disease prophylaxis and prevention
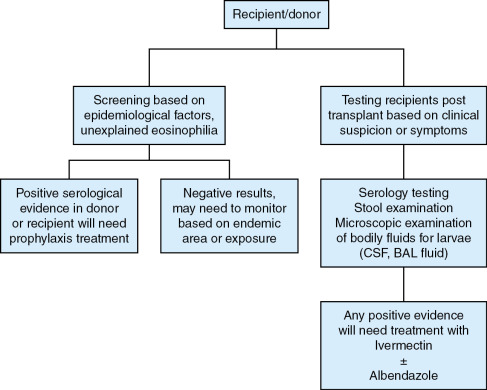
There are few existing guidelines suggesting the use of universal screening as it pertains to KTx programs. , , , However, reports based on surveys show that only 10% of organ procurement organizations actually screen for Strongyloides infection. Screening is based on the presence of epidemiologic risk factors, such as traveling to or having lived in an endemic area, unexplained eosinophilia, or a history of previous Strongyloides infection. However, geographic risk is not reliable enough to serve as the primary screening tool as adult worms can live up to 5 years. More robust screening algorithms are warranted to reduce the risk of donor-derived infections along with increased screening from organ procurement organizations. Depending on geographic location, transplant programs should perform Strongyloides screening universally. The Miami Transplant Institute has recently adopted a universal prophylaxis protocol given it experience with adults in whom donor-derived Strongyloides infections developed—one with a positive serologic test result and another with a false-negative serologic test result. If a screening test result is positive, the recipient should receive prophylaxis with ivermectin to reduce the risk after transplantation. Considerations to timing for prophylaxis must include assurance of ivermectin absorption. Therefore prophylaxis can occur during the pretransplant period or soon after transplantation, depending on recipient or donor seropositivity.
Diagnosis
The gold standard for diagnosis is isolation of larvae from stool specimens. However, the sensitivity of this method remains low, ranging from 15% to 30%. Serial stool examinations can be submitted to increase the sensitivity. Based on the life cycle of this organism, larvae may not be present during the time of stool examinations. Additionally, the gastrointestinal function of the individual needs to be considered. Recently serologic testing has become more popular and has become the method of choice for screening. , , Sensitivity varies depending on the assay performed; indirect immunofluorescence assays having the highest sensitivity. However, these are not commercially available. Sensitivity ranges from 80% up to 96% and specificity ranges from 90% up to 96%. , Serologic test results may remain positive even after appropriate treatment for Strongyloides infection . Experimentally, investigators have attempted to use immunoglobulin Ig A levels in saliva as an alternative, but serologic testing has higher sensitivity. Polymerase chain reaction (PCR) has been investigated as well and demonstrates potential, but it is not commercially available and the reliability needs to be evaluated in larger clinical trials. Eosinophilia may only be present in 30% of cases, but if present and unexplained, it should prompt screening immediately. An approach to risk-based screening and symptom-based diagnostic testing for Strongyloides in organ donors and organ recipients is shown in Fig. 32.1 .
Treatment
The drug of choice is ivermectin for the treatment of asymptomatic disease, hyperinfection, and disseminated disease. Treatment may be prolonged in immunocompromised patients. Prophylactic dosing is 200 μg/kg once daily for 2 days; some experts recommend repeating the dose 2 weeks apart. Albendazole is an alternative treatment for Strongyloidiasis infection for 3 to 7 days. However, some experts recommend the combination of ivermectin and albendazole in hyperinfection and disseminated disease in the SOT population. , Parenteral formulations are not approved and may not be available; however, the veterinary formulation may be used subcutaneously. The pediatric and adult dosing for ivermectin and albendazole is shown in Table 32.1 .
| Treatment Drug | Adult Dosing | Pediatric Dosing |
| Ivermectin | 200 μg/kg daily once a day for 1 to 2 days | < 15 kg: No dosing available ≥15 kg: 200 μg/kg daily once a day for 2 days |
| Albendazole | 400 mg twice a day | ≤ 10 kg: 200 mg a day >10 kg: 400 mg twice a day |
Infection prevention and anticipatory guidance
The best preventative effort to avoid infection is to wear shoes while walking on soil in endemic areas. Human-to-human transmission is extremely rare except in donor-derived infections in SOT recipients. Standard precautions such as hand hygiene after stool contact are recommended in hospitalized patients. Screening for Strongyloides infection should be performed in patients who will receive immunosuppressive therapy.
Cryptosporidium species
Epidemiology and risk factors
Cryptosporidium is an intracellular parasite that has become one of the leading causes of diarrheal disease worldwide. The first human Cryptosporidium infection was noted in 1976. Since that time, it has increasingly become recognized as a common cause for diarrheal disease, with the Centers for Disease Control and Prevention (CDC) estimating approximately 750,000 cases occur annually, only a fraction of which are reported. From 2001 to 2010, Cryptosporidium was the leading cause of all waterborne outbreaks of diarrheal disease in the United States. Per the CDC, Cryptosporidium -related disease hospitalizations cost $45.8 million dollars per year. Estimated Cryptosporidium seroprevalence in North America ranges from 25% to 35%. ,
Although there are many species of Cryptosporidium , the species that most commonly infect humans are C. parvum and C. hominis , with some studies noting that C. parvum causes nearly 97% of Cryptosporidium infections , C. hominis primarily causes human-to-human infection, whereas C. parvum can cause both human-to-human disease and animal-to-human infection. Cattle and sheep seem to serve as the primary animal reservoirs for human disease, with animal waste contaminating ground water. , ,
Fecal-to-oral transmission occurs via contaminated drinking water, food, recreational water, and indirectly via fomites. , , In the United States, Cryptosporidium oocysts are estimated to be in 55% to 87% of surface water tested. Individuals participating in recreational water activities are at increased risk for Cryptosporidium infection, with 90% of recreational outbreaks from 1991 to 2000 linked to swimming pools and water parks. Some studies report a seasonal increase from June to September, thought to be due to increased exposures to contaminated water via recreational activities. Exposure to contaminated drinking water has also led to many Cryptosporidium -related outbreaks; one of the largest was the 1993 Milwaukee outbreak, the in which nearly 400,000 individuals were affected. , , As an intracellular parasite, once Cryptosporidium oocysts are ingested, they infect intestinal epithelial cells. Cryptosporidium undergoes both asexual replication, resulting in merozoites that infect neighboring epithelial cells, and sexual replication, which results in oocysts. Once excreted, oocysts are immediately infectious and can lead to autoinfection and contamination of the immediate environment. Oocysts do not reproduce outside the host. Infection can result from a very small number of oocysts; some studies note as few as 10 oocysts result in infection. With an infected individual shedding up to 10 8 oocysts per day, outbreaks occur easily. ,
Although Cryptosporidium infections affect all people, both immunocompetent and immunocompromised, children and immunosuppressed individuals are at higher risk for disease. Children 2 to 11 years old are at increased risk for person-to-person transmission, with the highest number of cases in children younger than 5 years. , , Immunosuppressed individuals are also at increased risk for disease; one study showed Cryptosporidium spp. as the cause for 21% of diarrheal cases in SOT patients compared with only 3% of cases in the control group.
Clinical manifestations
The pathophysiology of Cryptosporidium infection is still unknown. The inflammatory response to intracellular infection leads to villous blunting and atrophy and increased cell permeability, which causes significant watery diarrhea and can contribute to malabsorption. , Rarely does bloody diarrhea occur and stool is often without fecal leukocytes. It is believed to be a secretory diarrhea process; however, no enterotoxin has been found. , Although the mechanism of infection remains unknown, it seems that the immune system’s ability to control Cryptosporidium infection is reliant on cell-mediated immunity. Given this information, transplant and oncologic patients are at significant risk for disease. , , The incubation period for Cryptosporidium is up to 2 weeks. Most infections in immunocompetent individuals are either asymptomatic or self-limited. ,
Clinical syndromes from Cryptosporidium infection can include acute infection, chronic infection, and fulminant disease. Acute infection often occurs in immunocompetent individuals, presenting primarily with watery diarrhea. About 90% of acute symptoms last 2 weeks, although symptoms can last up to 5 weeks. Chronic disease is classified as diarrheal symptoms lasting more than 2 months. Both acute and chronic infections can also be associated with abdominal pain, bloating, nausea, vomiting, and occasionally fever. Fulminant disease is most often seen in immunocompromised individuals and results in profuse, watery, cholera-like stool output, with liters of output per day, leading to significant concerns for hypovolemia. ,
Extra-intestinal symptoms can also occur with Cryptosporidium infection; these symptoms are mostly biliary and respiratory. Biliary disease, manifesting as right upper quadrant pain, nausea, and vomiting, was seen in up to 26% of patients with AIDS. Studies have shown that upper respiratory tract symptoms occur in 15% of pediatric cases. Symptoms manifest as bronchitis, cough, dyspnea, chest pain, and increased secretions. Studies from developing nations have linked cryptosporidiosis to decreased growth and physical development, as well as diminished cognitive development.
Disease prophylaxis and prevention
At present, there are no recommendations for screening of Cryptosporidium infection in immunocompromised patients. Testing is suggested only for patients with prolonged diarrheal symptoms. Prevention focuses on good hygiene and decreasing exposures to potential environmental contamination.
Diagnosis
Cryptosporidium oocysts are not generally found on routine ova and parasite testing; if there is concern for infection with Cryptosporidium, the laboratory should be notified of this concern. Often a modified acid-fast stain can be used to detect the oocysts in the stool sample. , , , Given that oocysts are only shed intermittently, a single stool sample has an estimated sensitivity of about 30%; therefore multiple stools samples on multiple days are suggested to increase sensitivity of testing. , Most sources would recommend a minimum of three stool samples. Direct fluorescent antibody testing, enzyme immunoassays, and PCR testing are now more commonly used for diagnosis because they have increased sensitivity compared with microscopic testing; some studies report near 100% sensitivity and specificity with these newer methods. , Point-of-care rapid antigen testing is also available.
Treatment
Currently there are very few effective treatments for Cryptosporidium infection. For immunocompetent individuals, supportive care alone is often sufficient. In 2005, the U.S. Food and Drug Administration (FDA) approved nitazoxanide as a treatment for Cryptosporidium infection; it is the only FDA-approved treatment currently available. Even with this as approved treatment, efficacy is still in question, particularly in immunocompromised patients.
An Egyptian study compared 3 days of nitazoxanide treatment versus placebo in immunocompetent individuals with C. parvum infection. Treatment with nitazoxanide significantly reduced symptoms; 80% of the patients had resolution of symptoms at the 7-day follow-up compared with only 20% in the placebo group. Additionally, 67% of patients in the treatment arm had no oocysts present in the posttreatment stool samples compared with only 22% in the placebo group. , Another study comparing nitazoxanide treatment versus placebo in Zambian HIV-infected and HIV-uninfected children showed improved symptoms and improved parasitologic findings in the HIV-uninfected children treated with nitazoxanide versus those who received placebo; however, this significance was not seen in the HIV-infected children treated with nitazoxanide. , , ,
Current recommendations suggest a 3-day course of nitazoxanide for immunocompetent individuals, with a minimum of 14 days of treatment for those who are immunosuppressed. , If nitazoxanide is not available, paromomycin is an alternative treatment option, although the limited data available show mixed results regarding efficacy. , Additionally, there is scant literature on combination therapy—paromomycin plus azithromycin—in the treatment of Cryptosporidium infection in HIV populations, showing some success, particularly for chronic cryptosporidiosis. , Other case studies of treatment in immunosuppressed patients suggest that decreased immunosuppression and combination therapy resulted in improvement in clinical course.
Adjunct treatment with oral human immunoglobulin and immune bovine colostrum have also been evaluated in various case reports, showing that these treatments may attenuate clinical infection, but there are no trials evaluating these treatment options. , Additional studies on effective treatments for Cryptosporidium infections are needed.
Infection prevention and anticipatory guidance
Cryptosporidium oocysts are hardy organisms and are able to survive in the environment for long periods of time. Most standard disinfectants and cleaners are not effective against Cryptosporidium spp.; therefore hydrogen peroxide and bleach are the preferred disinfectants for Cryptosporidium in the health care setting. Additionally, alcohol-based hand sanitizers are ineffective and soap and water is the preferred hand hygiene method. In the health care setting, standard precautions should be used for all patients with Cryptosporidium infections. For those who are diapered or incontinent of stool, contact precautions should also be implemented.
Outside the healthcare setting, good hygiene is the best method of prevention. Both foodborne-related outbreaks and animal-to-human transmission can be lessened by practicing good hand hygiene. Given the link between Cryptosporidium and waterborne outbreaks from various water sources, participation in recreational water activities should be done with caution. For those recovering from infection with Cryptosporidium , participation in recreational water activities should be avoided until at least 2 weeks after diarrheal symptoms resolve to help prevent contaminating the water. Individuals should also avoid ingestion of recreational water or water from any untreated water source. If an individual is in an area with unsafe water, boiling and filtration can help mitigate risk. International travel has also been associated with increased risk for infection, particularly when traveling to locations without safe water sources. Immunocompromised individuals should be aware of this risk and take precautionary steps where appropriate. ,
Chagas disease
Epidemiology and risk factors
Human trypanosomiasis in the Americas, also known as Chagas disease (CD), is caused by Trypanosoma cruzi . It is endemic to the regions of Mexico and Central and South America. , It is estimated that 65 million people are at risk and 7 to 8 million people are infected in Latin America. , Because of immigration and international travel, it is estimated that more than 300,000 people living in the United States are infected with T. cruzi. , Therefore Chagas disease is becoming an emerging infectious disease in the United States. Triatomine bugs are responsible for vector-related transmissions. After initial infection, individuals enter the acute phase of infection characterized by mild febrile illness or nonspecific signs and symptoms. Most infected people are asymptomatic. In endemic areas, there is a high level of parasitemia during the acute phase. After a few months, infected people progress to the chronic phase without symptoms. However, 20% to 40% of people in the chronic phase develop cardiac, gastrointestinal, and rarely, peripheral nervous system disease. , , Cardiomyopathy is the most recognized complications during the chronic phase, necessitating heart transplantation (HTx). During the chronic phase, approximately 60% to 80% of patients enter a clinical latency, which is referred to as the indeterminate form. , During the acute phase, blood smear or buffy coat testing can be used to look for parasites. However, during the chronic phase, in particular during the indeterminate form, diagnoses can only be made through serologic testing. ,
Vector-related, oral, and vertical transmission are the most common routes of infection in endemic areas, whereas blood transfusion and SOT are most commonly associated with transmission in nonendemic areas. , , The increasing number of people traveling to and from endemic areas has increased the pool of donors who are infected with CD. , , The rate of seropositivity in the United States approaches 0.9%; however. the rate can be affected by geographic location and population demographics. , Studies in transplant candidates from Central America have demonstrated seropositivity rates of 4.5%. In the United States, Florida and California account for more than 50% of seropositive cases for CD.
As of 2016, 14 cases of donor-derived infections were documented in United States with a 13% to 18% rate of transmission reported. , However, in HTx the rate of donor-derived CD owing to reactivation of the parasite within the cardiac allograft may be as high as 61% within the first 24 months after transplantation. The rate of transmission in KTx is between 8% and 22%; the rate in liver transplant is between 20% and 22%; and in HTx the rate is approximately 75%. , , Recipient-negative and donor-positive (R − /D + ) patients have a low risk of donor derived infection whereas R + /D − patients may have a 33% risk of reactivation of prior infection within the recipient. Few cases have been reported in bone marrow transplant recipients. Risk factors leading to a positive serologic test result are being born in Latin America, born to a mother from Latin America, living in mud houses, receipt of unscreened blood transfusion, or residence in an endemic area from 3 to 6 months. , , , Demographics for patients treated under an investigation new drug program from the CDC showed a few pediatric cases including a congenital infection. These data suggest that pediatric screening may also be warranted and that clinicians caring for pediatric organ candidates and recipients should likely follow adult guidelines. Most guidelines suggest against accepting heart or intestine allografts from a positive donor but support acceptance of kidney, pancreas, liver, and lung allografts. Risk/benefit analysis must be performed to accept organs from positive donors as transmission is not extremely high.
Clinical manifestations
During the chronic stage of CD, immunocompetent hosts can present with cardiac arrhythmias, progressive heart failure, and/or segmental dilatation of the gastrointestinal tract with megaesophagus or megacolon. , CD can manifest as myocarditis leading to allograft dysfunction, with rapid onset of congestive heart failure in HTx recipients. Other symptoms may include fever, inflammatory panniculitis, and skin nodules. In other organs, CD may present with fever, atypical skin lesions, myocarditis, or meningoencephalitis. In some cases, the skin lesions resemble erythema nodosum. In CD, mortality has been reported as low as 0.7%, with other studies suggesting early detection and treatment leading to favorable outcomes.
Disease prophylaxis and prevention
There are few guidelines suggesting universal screening in endemic areas with serologic laboratory tests. In the United States, only 19% of organ procurement organizations screen for CD, either universally or based on epidemiologic risk factors, such as being born in Latin America, born to a mother from Latin America, living in mud houses, receipt of unscreened blood transfusion, or residence in an endemic area from 3 to 6 months. , , , There are no randomized controlled trials to evaluate the efficacy of prophylaxis in the prevention of CD. Case reports on the use of prophylaxis to prevent transmission in living related transplant programs as well posttransplant prophylaxis do not provide sufficient evidence to inform recommendations either way. , , Some programs suggest screening after transplant to inform treatment instead of providing prophylaxis. In Brazil, an endemic area, transplant programs do not routinely provide prophylaxis. Additionally, there are reports that use of mycophenolate mofetil and corticosteroids increases the risk of CD. , , , However, there is no consensus on which immunosuppressive regimens should be used in a transplant recipient with a positive donor. , Current guidelines are hindered by a lack of widespread data collection. Therefore there is little consensus relating to screening recipients and donors, prophylaxis questions, and immunosuppressive regimens.
Diagnosis
During the acute phase of CD, the use of microscopic examination for parasites using blood smear or buffy coat is the optimal laboratory examination. Biopsy specimens can be submitted for histopathology and PCR-based testing. However, for screening purposes one serologic test is sufficient to diagnose the chronic phase of CD. For a clinical diagnosis, two separate antigen and/or techniques are needed. There are three distinct methodologies that can be performed: enzyme-linked immunosorbent assay, immunofluorescence assay, and radioimmunoprecipitation assay. Currently two companies have FDA approval for donor screening using enzyme-linked immunosorbent assay kits. Overall sensitivity is 99% to 100% and specificity is 98% to 100%. , PCR is not routinely used for screening donors as it would miss patients in the chronic phase. , PCR is the preferred methodology for posttransplantation screening for CD. Blood PCR may lead to early detection before the onset of clinical symptoms as it is interpreted as parasitemia. , However, there are some studies suggesting specificity may be lower and some experts would recommend to wait for clinical symptoms. In postmortem organs examined by PCR, T. cruzi was detected by PCR and may explain transmission to the recipient. PCR may not be readily available commercially; however, it is reliably available from the CDC. For patients screened based on epidemiologic risk factors with a positive serologic screening result before transplant, follow-up after transplant should include PCR and microscopic examination every week for the first 2 months, then every 2 weeks during the third month, and monthly until 6 months after transplant. , However, recently programs have adopted a modified posttransplant screening process based on specific organs. For HTx patients, the process is weekly PCR and microscopic examination weekly during the first 2 months, then every other week for months 3 through 12, every 3 weeks for months 13 through 24, and every 6 months thereafter. For non-HTx patients, the process is weekly during the first 2 months, then every other week for months 3 through 6, and then annually thereafter.
Treatment
The drug of choice is benznidazole for the treatment of CD. , , , Benznidazole is available commercially and is approved by the FDA for children as young as 2 years. Treatment is prolonged in immunocompromised patients up to 60 days with negative test results documented for clearance. Nifurtimox is an alternative treatment for CD and is only available through an investigational new drug program from the CDC. , Nifurtimox is not FDA approved for the treatment of CD and the length of therapy is approximately 90 days with a negative test result indicating cure. Patients should be monitored annually post treatment to assess for potential reactivation. The pediatric and adult dosages of the medications are shown in Table 32.2 .