© Springer International Publishing AG 2018
Louise Olsson (ed.)Timely Diagnosis of Colorectal Cancerhttps://doi.org/10.1007/978-3-319-65286-3_66. Standardised Investigation and Referral of Alarm Symptoms for Colorectal Cancer
(1)
School of Public Health, University of Sydney, New, South Wales, Australia
(2)
RPA Institute of Academic Surgery and Surgical Outcomes Research Centre (SOuRCe), Sydney Local Health District, New South Wales, Australia
(3)
Central Clinical School, University of Sydney, New South Wales, Australia
Key Points
More timely diagnosis of symptomatic cancer could identify tumours at an earlier stage when treatment is more likely to be effective, thereby improving cancer outcomes
Standardised referral pathways and ‘fast track’ programs aim to reduce the time interval between a patient’s presentation to the health care system with a suspicious symptom and cancer diagnosis (the ‘diagnostic interval’)
Suspicious colorectal symptoms are not strongly associated with colorectal cancer.
Standardised referral pathways and ‘fast track’ programs are complex to evaluate; to date there is no clear evidence of effectiveness to reduce cancer burden in the population.
In recent years, several countries have introduced standardised investigation and referral pathways for people who present in primary care with symptoms that are suspicious for cancer. This chapter provides an overview of standardised approaches in terms of the underlying rationale, the international experience and the complexity that is inherent in evaluating the impact on cancer outcomes in the population.
6.1 Impetus for Standardized Referral and Investigation Pathways
One of the major driving forces behind national cancer policies to standardise the assessment of people with suspicious symptoms for cancer is the marked disparities in cancer outcomes between countries that have been apparent over the past 20 years. In the mid-1990s, the EUROCARE Study was established within the European Union as a large, collaborative research project to analyse survival data obtained from cancer registries using standardized protocols. The purpose was to investigate trends in cancer survival over time as well as regional variations [1]. EUROCARE investigators reported marked differences in 5-year relative survival between countries for most cancers, including colorectal cancer. For example, during the period 1978–1985, for patients aged 60–69 who had colon cancer, the mean relative survival among the 21 populations studied was 40% but this varied from over 50% in Switzerland and the Netherlands, to 22% in Poland. Some countries, namely the United Kingdom (29–39%) and Denmark (42%), had considerably lower survival rates than comparable neighbours [2].
Subsequently there have been a number of iterations of EUROCARE to include more comprehensive and complete information about cancer cases across Europe, and several in-depth analyses have been undertaken to try to better understand these disparities. Differences in the stage of cancer at diagnosis have been consistently identified as one of the main drivers for the differences in outcome [3]. Over the ensuing years, further iterations of EUROCARE have demonstrated gradual improvements in 5-year relative survival across most regions and for most cancers, although the rate of improvement has been highly variable. For colorectal cancer, by 2007, the mean 5-year relative survival for Europe had improved to 57% for colon cancer and nearly 56% for rectal cancer, but with the UK and Ireland continuing to have among the lowest rates for colon cancer (51.8%) while ranking towards the middle (53.7%) for rectal cancer [4].
Extending the international comparisons beyond Europe, the International Cancer Benchmarking Partnership (ICBP) similarly reported substantial geographic differences in 1- and 5-year relative survival across a number of cancer types, with rates consistently higher in Canada, Australia and Sweden, intermediate in Norway and lowest in Denmark, the United Kingdom and Ireland [5].
These and other studies stimulated much debate about the possible reasons for the apparent disparities. While differences between countries in the proportion of patients diagnosed with more advanced disease is an important explanation and has been the focus of policy responses, other factors could include methodological differences between different cancer registries, regional differences in tumour biology, the comorbidity burden in the different populations, differences in access to treatment services and variations in the quality of cancer care provided [6].
Despite these international differences in cancer survival, in all populations there is a strong association between earlier stage of colorectal cancer at diagnosis and improved survival. In many regions, the 5-year relative survival for Stage 1 disease is over 90% compared with 40–60% for those with nodal involvement and around 10% or less for those with metastatic disease at diagnosis [7]. The self-evident desirability of diagnosing colorectal cancer at an earlier stage has focused attention on the timeliness of cancer diagnostic pathways and whether there are any causes of delay that could be rectified so as to improve patient outcomes.
While there is consensus that earlier diagnosis of colorectal cancer is paramount, the impact of the timeliness of cancer diagnosis on cancer outcomes has been the subject of some debate [8, 9]. If the development, growth and spread of a tumour follows a linear path, then earlier diagnosis should identify earlier-stage cancers. However, tumour biology may be more complex. For example, it is possible that some tumours develop and spread as a result of previous irreversible genetic mutations that may not follow a predictable timeline [10]. Furthermore, even if the linear path were the predominant natural history, the timelines and likely duration of a ‘window of opportunity’ for curative treatment are far from clear. It is likely that efforts to improve the timeliness of diagnosis by days or weeks would be inconsequential if the time for development of the tumour took months or years.
For patients with symptoms that are suspicious for cancer, the pathway to diagnosis is complex, comprising a number of varying time intervals including the time from first becoming aware of a symptom to seeking medical advice and the time from first clinical presentation to definitive diagnostic testing. In order to standardize the measurement of diagnostic intervals between different studies of early cancer diagnosis, the Aarhus statement [11] has been developed by an international team to provide consistent definitions and methods. The ‘diagnostic interval’ has been defined as the time interval between the date of first presentation to the health care system to the date of cancer diagnosis.
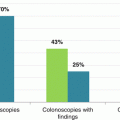
A standardized approach to defining and measuring cancer diagnosis pathways is particularly important as much of the evidence informing the debate around early cancer diagnosis is derived from observational studies rather than randomised trials [10]. In a 2010 national survey of patients with cancer in England, 32% of those with colon cancer and 23% of those with rectal cancer had had three or more consultations with their general practitioner for cancer symptoms before they were referred to a hospital for investigation, suggesting there may be opportunities for earlier intervention in a substantial proportion of patients [12]. Other studies have investigated the time from presentation with suspicious symptoms to colorectal cancer diagnosis, with highly variable findings. For example, a Danish prospective, population-based study (n = 268) reported a median diagnostic interval of 40 days, but this varied between patients with alarm symptoms (37 days) and those with vague symptoms (74 days) [8]. In the UK, an analysis of 6557 general practice records for people with colorectal cancer found the mean diagnostic interval was 120 days (median 80 days), with significantly longer intervals for those with vague symptoms, older people and women [13].
There have been conflicting findings from studies investigating the relationship between the length of the diagnostic interval and two important outcomes, namely stage at diagnosis (often used as a proxy for cancer survival) and survival from colorectal cancer. In two systematic reviews, Ramos and colleagues found no association between the diagnostic interval and stage at diagnosis or cancer survival, although there were some suggestions of differences between colon and rectal cancer for cancer stage [14, 15]. However a more recent systematic review found that for colorectal cancer, the weight of evidence tended towards earlier stage at diagnosis and improved survival for shorter diagnostic and therapeutic intervals [16].
Furthermore, the relationship between the diagnostic interval and survival for people with symptomatic cancer may not be unidirectional or linear. Some studies have demonstrated that for people with highly suspicious or ‘alarm’ symptoms, a shorter diagnostic interval paradoxically is associated with poorer survival whereas the reverse is apparent for those with vaguer symptoms. This could be explained the different presentation and behaviour of aggressive and more insidious tumours. People with highly aggressive disease could develop more ‘high risk’ or severe symptoms, thereby triggering faster diagnosis but this would have little impact on subsequent survival due to the underlying biology of the cancer [10].
Indirect evidence of the relationship between more timely cancer diagnosis and improved outcomes at a regional level is also apparent in an ecological analysis conducted as part of the International Cancer Benchmarking Partnership. Case scenarios were used to investigate general practitioners’ readiness to refer a patient with suspicious symptoms for definitive diagnostic investigation. This study found a positive correlation between earlier referral and 1-year relative survival for the 11 participating regions in Scandinavia, Australia, Canada and the United Kingdom [17].
For it to be possible to reduce the diagnostic interval for symptomatic colorectal cancer, there must be suspicious symptoms or other clinical ‘alarm’ features that are good predictors of cancer and therefore flag that a patient is in a higher risk category. Common ‘alarm’ features for colorectal cancer include rectal bleeding, a change in bowel habit, iron deficiency anaemia and abdominal or pelvic mass. However, it is well recognised that several of these factors are not unique to colorectal cancer. General practitioners have the difficult task of urgently triaging patients who have serious disease whilst not over-investigating those with benign causes, often on the basis of vague symptoms. Furthermore, in many health care systems, general practitioners act as the gatekeepers to diagnostic services and specialist care, thereby taking responsibility for appropriate and efficient use of limited health resources.
Unfortunately, most studies of specific symptoms have found relatively poor associations with colorectal cancer. One systematic review of 15 primary studies concluded that that most alarm features had poor sensitivity but moderate to good specificity [18, 19]. The development of more sophisticated risk stratification models based on a combination of symptoms, clinical signs and other factors, such as patients’ age and sex are likely to improve sensitivity and specificity and thereby better identify individuals who should be referred urgently.
6.2 Introduction of Standardised Approaches to Investigation of Suspicious Symptoms
In the early 2000s, several countries, notably the UK and Denmark, responded to the emerging international data on marked disparities in cancer outcome to introduce policies to accelerate and streamline patient pathways for cancer diagnosis and treatment. While there are differences in approach in different countries, consistent components include identifying an agreed set of ‘alarm’ symptoms and the development of policy or guidelines about the nature and timeliness of subsequent referral for further investigation.
6.2.1 The Danish Approach
In response to growing concern about long waiting times, in 2001 the Danish government passed legislation mandating that the waiting period between a diagnosis of cancer and commencement of treatment was to be no longer than 2 weeks [20]. However, this did not address waiting times in the pathway to diagnosis. In 2005, a national cancer plan identified the development and introduction of standardised cancer patient pathways as a key strategy to reduce delays in the diagnosis as well as the treatment of cancer [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree