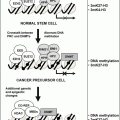
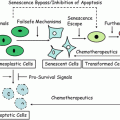
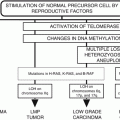
Fig. 21.1
Clinical outcomes of HPV vaccination trials in an intention-to-treat meta-analysis. Adapted from [35].
Another form of cancer with an infectious etiology is gastric cancer. Helicobacter pylori (H. pylori) infection is the leading cause of gastric cancer worldwide. Infection with this ubiquitous infectious agent is harbored by approximately half of the population worldwide [24]. Malignant transformation is achieved through a multistep cascade including chronic inflammation, atrophy, metaplasia, dysplasia, and invasive cancer. Interestingly, the target cells of malignant transformation in H. pylori caused gastric cancer are bone marrow derived tissue stem cells. These cells are infected in bone marrow and recruited into gastric tissue after local atrophy due to chronic inflammation acting then locally as cancer stem cells. In view of the high prevalence of H. pylori infection and gastric cancer, it is clear that powerful modulatory factors exert their influence. However, the exact nature of these modulatory effects is unknown. Immunologic competence and genetic susceptibility of individuals as well as genetic variation of H. pylori DNA, for example, may play a role in the etiology of both atrophic gastritis and gastric cancer. In a study of 104 affected individuals from Costa Rica, one of the countries with the highest gastric cancer incidence and mortality rates in the world, expression of the H. pylori genes vacA s1b and vacA m1 was associated with gastric cancer and the expression of vacA m1 was associated with atrophic gastritis [39].
21.3.2 Diet
The role of dietary factors in the development of cancer is controversial. While many researchers and lay people instinctively believe that diet—being one of the most basic aspects of life and affecting all organ systems—is very likely to affect cancerogenesis, study data are controversial. For example, a prospective Swedish cohort study of 10,564 men with a mean follow-up of 11 years found no association between dietary intake of total, saturated, or monounsaturated fat, and risk of prostate cancer [40]. A nationwide study from Sweden found no reduced risk of breast cancer in women with regular fish consumption [41]. The prospective National Institutes of Health-AARP Diet and Health Study investigated grain and fiber intake of 291,988 men and 197,623 women aged 50–71 years. Total dietary fiber intake was not associated with colorectal cancer. However, whole-grain intake was inversely associated with a moderately reduced colorectal cancer risk (Relative Risk 0.79; 95 % Confidence Interval 0.70–0.89) [42]. Other studies found an association between monounsaturated and polyunsaturated fatty acid intake and breast cancer. A population-based prospective cohort study including 61,471 women reported an inverse association with monounsaturated fat intake and a positive association between polyunsaturated fat intake and breast cancer. The reduction of relative risk for each 10-g increment in the daily intake of monounsaturated fat was 0.45 (95 % Confidence Interval 0.22–0.9) [43]. In accordance, a prospective cohort study of 90,655 premenopausal women, recruited within the Nurses’ Health Study, found that the dietary intake of animal fat, mainly from red meat and high-fat dairy foods, during the premenopausal years is associated with an increased risk of breast cancer [44]. Unfortunately, dietary modification aimed at reducing dietary fat has not led to a reduced incidence of breast cancer and colon cancer in a large randomized trial of 48,835 postmenopausal women [45, 46]. In accordance, a controlled dietary modification was also insufficient to significantly reduce the recurrence risk in a randomized trial of 3088 early breast cancer survivors [47]. Specifically, adoption of a diet that was very high in vegetables, fruit, and fiber, and low in fat did not reduce additional breast cancer events or mortality during a 7.3-year follow-up period. On the other hand, the randomized Women’s Intervention Nutrition Study, launched in 1987, found a reduction of breast cancer recurrences among estrogen receptor-negative disease [48]. This randomized trial of 2437 women with early-stage breast cancer determined that low-fat dietary interventions can influence body weight and decrease breast cancer recurrence. Dietary fat intake was lower in the intervention than in the control group and relapse rates were 12.4 % in the control group versus 9.8 % women in the dietary group. However, this effect was restricted to estrogen receptor-negative disease. In summary, no clear and concise dietary strategy has emerged for the primary or secondary prevention of breast cancer.
Other dietary compounds potentially affecting cancer development are phytoestrogens found for example in soy. The sustained consumption of phytoestrogen-rich food correlates with a reduced incidence of breast cancer among Asians. Of note, this association has not been found in European and US populations [49]. Human dietary intervention trials have noted a direct relationship between phytoestrogen intake and a favorable hormonal profile associated with a decreased breast cancer risk. According to a systematic review, 22 case–control and cohort studies examined the incidence of breast cancer among women with and without a diet high in phytoestrogens. A meta-analysis of 21 of these studies among Asian populations found a significantly reduced incidence of breast cancer among past phytoestrogen users. Some, but not all randomized controlled trials document a beneficial effects of phytoestrogens on surrogate parameters such as bone mineral density, vasodilation, platelet aggregation, insulin resistance, and serum concentrations of triglycerides, high-density lipoprotein, and low-density lipoprotein. However, none of the available randomized controlled trials documents a protective effect of phytoestrogens for the clinical end point of site-specific cancer or overall cancer incidence [50]. No consistent evidence of an inverse association between phytoestrogen consumption and breast cancer incidence has been documented among US and European women [49, 50]. Epidemiologic and rodent studies suggest that breast cancer chemoprevention by dietary phytoestrogen compounds may be dependent on the ingestion of large amounts of phytoestrogens before puberty, when the mammary gland is relatively immature [49].
Some dietary components such as polyphenols in tea beverages may affect cancerogenesis, but the evidence supporting this assumption is controversial. Indirect evidence from epidemiologic studies suggests that the incidence of prostate cancer may be lower in populations with regular tea consumption [51].
Adherence to a Mediterranean diet has been associated in observational studies with a reduced incidence and mortality of some cancers. In a meta-analysis of 21 cohort studies including 1,368,736 subjects and 12 case–control studies with 62,725 subjects, adherence to a Mediterranean diet resulted in a significant reduction of overall cancer mortality (10 % reduction) and colorectal cancer (14 % reduction), prostate cancer (4 % reduction), and aerodigestive cancer (56 % reduction) incidence. Nonsignificant changes could be observed for breast cancer, gastric cancer, and pancreatic cancer [52]. However, there is no controlled evidence regarding a cancer-preventing effect of the Mediterranean diet. Thus, it remains unclear, what the exact dietary strategy for cancer prevention should be and whether or not diet is an independent factor or works only within certain cultural, genetic, or climate conditions.
21.3.3 Alcohol
Alcohol features prominently among dietary factors associated with sporadic cancer incidence. However, the biologic effect of alcohol on cancerogenesis is unclear. It has been hypothesized that one mechanism implicated in alcohol-related carcinogenesis may be oxidative stress from alcohol metabolism, inflammation, and increased iron storage. Ethanol-induced cytochrome P-450 2E1 produces various reactive oxygen species, leading to the formation of lipid peroxides such as 4-hydroxy-nonenal. Furthermore, alcohol impairs the antioxidant defense system, resulting in mitochondrial damage and apoptosis. Chronic alcohol exposure elicits tissue hyperregeneration due to the activation of tissue-specific survival factors and interference with the retinoid metabolism. In addition, direct DNA damage may result from acetaldehyde, which can bind to DNA, inhibit DNA repair systems, and lead to the formation of carcinogenic exocyclic DNA adducts. Finally, chronic alcohol abuse interferes with methyl group transfer mechanisms and may thereby alter gene expression [53].
A wide variety of cancers are found at an increased incidence among chronic alcohol users. In case–control studies, the association is dose-dependent, but varies with different cancer sites. Associations between alcohol consumption and an increased risk of cancer have been reported for breast, lung, and ovarian cancer in case–control and prospective cohort studies.
For example, consumption of 20 g or more of alcohol per day increases the risk of breast cancer by 70 % compared to nondrinkers. It is of note that even moderate levels of alcohol intake, i.e., 8 g per day, are sufficient to increase the risk of breast cancer by 50 % [54]. In a large prospective Danish cohort of 13,074 women, an alcohol intake of more than 27 drinks per week increased breast cancer risk more than threefold among premenopausal women irrespective of alcohol type. In postmenopausal women, an intake of more than six spirits per week increased breast cancer risk over twofold [55]. This effect may be limited to estrogen receptor positive breast cancers and may also be enhanced by the concomitant use of hormone replacement therapy [56]. Carpenter et al. described a statistically significant 1.8-fold increased risk of lung cancer for one or more drinks of hard liquor per day from ages 30 to 40 years after adjusting for other risk factors such as smoking [57]. Serous invasive ovarian cancer has also been associated with alcohol consumption. Interestingly, this effect was restricted to early adult regular drinking suggesting an increased susceptibility of ovarian epithelial tissue to alcohol-induced genetic damage during the early reproductive phase [58].
21.3.4 Radiation
The cancerogenic effect of ionizing radiation based on increased mutagenesis, DNA adducts, and chromosome strand breaks has been established since the early twentieth century. Chronic low-dose exposure, as well as acute high-dose exposure, to ionizing radiation increases the incidence of various forms of malignancies including solid cancers. In a long-term study of 105,448 survivors of the Hiroshima and Nagasaki nuclear bomb explosions, 17,448 first primary cancers were diagnosed between 1958 and 1998 [59]. Solid cancer rates increased by 35 % per Gray for men, and by 58 % per Gray for women at age 70, after exposure at age 30. Significant radiation-associated increases in risk were seen for most sites including oral cavity, esophagus, stomach, colon, liver, lung, non-melanoma skin, breast, ovary, bladder, nervous system, and thyroid, as well as all histologic subgroups of these cancers.
Nuclear power plant accidents such as those in Three Mile Island, Chernobyl, and Fukushima are associated with different cancer risks compared to atomic bomb explosions. In the region around Chernobyl, for example, more than five million people may have been exposed to excess radiation, mainly through contamination by iodine-131 and cesium isotopes. Interestingly, studies evaluating leukemia and non-thyroid solid cancers have not shown consistently elevated risks in the regions around Chernobyl. In the population around Three Mile Island, there was a notable temporary increase in cancer diagnoses in the years immediately after the accident, but this increase may have been the result of intensified cancer screening in the area. Long-term follow-up has shown no increases in cancer mortality [60].
Low-dose long-term exposure also leads to a significant increase in solid cancer risk, as demonstrated by the Techa River cohort study [61]. This investigation of 446,588 person-years of individuals exposed to environmental radiation releases associated with a Soviet nuclear weapons facility in the Southern Urals found a significant dose response with 3 % of solid cancers attributable to radiation exposure.
Another form of electromagnetic waves, i.e., ultraviolet (UV) radiation, has also been associated with sporadic cancer risk. Chronic exposure to solar UV radiation is the most important risk factor of non-melanoma skin cancer [62]. The typical UV-induced DNA damage consists of the generation of dimeric photoproducts between adjacent pyrimidine bases. Both UV-A and UV-B radiation induce pyrimidine dimers and promote the production of oxygen and nitrogen species with subsequent damage to DNA, proteins, and lipids. One of the typical genetic targets of UV radiation-induced mutations is the tumor suppressor gene p53. In addition, UV radiation has an immunosuppressive effect adding to its carcinogenic activity [62]. UV radiation also promotes the development of cutaneous malignant melanoma, but the mechanisms of this effect are less clear.
Surprisingly, UV radiation also has cancer protective effects. Of note, nearly 20 types of cancer have been found to be inversely associated with solar UV-B exposure. In a meta-analysis of second cancers after non-melanoma skin cancer, the risk of subsequent colon, gastric, rectal, cervical, and esophageal cancers was significantly reduced [63]. These data suggest that UV-B exposure may be protective against many internal cancers possibly due to increased vitamin D production.
21.3.5 Chemical Agents and Smoking
Exposure to chemical agents is associated with a wide variety of sporadic cancers. Polycyclic aromatic hydrocarbons and benzopyrenes, for example, induce cancer and sarcoma in murine models [64, 65]. These chemical agents may enter the body directly via environmental exposure or via specific vehicles such as cigarette smoke. Cigarette smoke contains potent carcinogens such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, benzopyrene, and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine [66]. In a case–control study of nine cancer centers from six European countries, smoking cigarettes at any time was associated with a fivefold increase in lung cancer risk and current smoking increased the risk ninefold. The association was dose-dependent and time-dependent. A significant excess risk of 70 % was associated with every ten pack-years smoked. After 10 years of smoking cessation, the relative risk decreased to 20 % compared to current smokers. Adverse characteristics were inhalation of smoke, smoking non-filter cigarettes, smoking dark-type cigarettes, and starting smoking at young age. The typical histologic type of lung cancer associated with cigarette smoke was small-cell lung cancer. In absolute terms, the proportion of lung cancer cases in the population attributable to cigarette smoking varied strongly, ranging from 14 % to 85 % [67].
Active and passive cigarette smoking is associated with a significantly increased risk of lung cancer as well as other forms of cancer, e.g., bladder cancer and head and neck cancer. In a prospective cohort study of 120,852 adult subjects, current cigarette smokers had a threefold higher bladder cancer risk compared to nonsmokers. Ex-smokers also experienced a twofold increased risk. In absolute terms, half of all cases of male bladder cancer and one-fifth of female bladder cancer were attributable to cigarette smoking [68]. In a prospective cohort study of 476,211 individuals, cigarette smoking was a strong risk factor of head and neck cancer with hazard ratios of 12.9 in women and 5.4 in men. Ever-smoking accounted for 45 % of head and neck cancers in men and 75 % in women, suggesting causality [69].
Asbestos is a chemical carcinogen prominent due to its past widespread use in fire protection devices in industry and construction. One of the malignancies associated with asbestos exposure is mesothelioma. In a South East English study of 5753 affected individuals, the highest incidence rates of mesothelioma were found along the Thames river and its estuaries reflecting areas of asbestos use in shipbuilding and industry in the past [70].
Cadmium is a toxic metallic trace element which is capable of blocking oxidative phosphorylation. Exposure to environmental cadmium increases cancer incidence and cancer-specific mortality. In a 20-year longitudinal study of Japanese living in a cadmium-polluted area, cancer-specific mortality was more than doubled (Rate Ratio 2.58; 95 % Confidence Interval 1.25–5.36) [71].
Chemical agents may influence carcinogenesis by inducing chronic inflammation. In an Australian case–control study of 1576 women with invasive and low malignant potential ovarian cancers and 1509 population-based controls, the use of talcum powder in the pelvic region was associated with a small, but statistically significantly increased risk [72].
21.3.6 Hormonal Factors
Hormonal factors play a major role in the development of sporadic cancer. Both cancer-promoting and cancer-preventing effects have been described for a variety of hormones and a variety of cancers. One of the most widely studied cancers with respect to the etiologic role of hormones is breast cancer. Epidemiologic studies link several factors related to estrogen production to an increased risk of breast cancer. These include early menarche, late menopause, obesity, use of postmenopausal hormone therapy, and plasma estradiol levels. Estrogens and estrogen metabolites may mediate breast cancer via estrogen receptor-mediated stimulation of breast cell proliferation with a concomitant enhanced rate of mutations or via genotoxic estradiol metabolites with a resulting increase in DNA mutations. Estradiol metabolites can cause DNA damage by formation of estradiol-adenine-guanine adducts which are released from the DNA backbone leaving depurinated sites which undergo error prone DNA repair. In addition, mutations and oxygen free radicals may be caused by redox cycling of 4-OH estradiol to the 3–4 estradiol quinone and back conversion to 4-OH estradiol. If one or both pathways are operative, sufficient numbers of mutations accumulate over a long period of time to induce neoplastic transformation [73]. From a clinical perspective combined estrogen/progestogen supplementation use has been demonstrated to significantly increase the incidence of breast cancer in a large randomized trial, the WHI study [74]. Of note, this was not the case when estrogen, i.e., conjugated equine estrogens, was used as a monotherapy [14]. Thus, the combination of exogenous estrogens and progestogens is cancerogenic, a fact that has been attributed to interactions between progestogens and the RANKL (receptor activator of NF-κB ligand)/RANK system [75]. It has been demonstrated that the in vivo administration of the progestogen medroxyprogesterone acetate triggers induction of the key osteoclast differentiation factor RANKL in mammary-gland epithelial cells. Genetic inactivation of the RANKL receptor RANK in mammary-gland epithelial cells prevented MPA-induced epithelial proliferation.
Antiestrogens such as tamoxifen and raloxifen have been shown to effectively prevent breast cancer in randomized trials [76]. Estrogens and antiestrogens also appear to play a role in other cancers, for example in lung cancer. In a Canadian case–control study, exposure to antiestrogens was associated with a significantly decreased mortality in those exposed both before and after the diagnosis of non-small-cell lung cancer [77].
Another line of evidence highlights an emerging role of estrogens in the initiation and progression of different malignancies through their interaction with stem cells [78]. Estrogens are involved in increasing hematopoietic stem cell self-renewal in female subjects and more specifically during pregnancy. It is likely that normal and tumor thyroid tissues, which express estrogen receptors, could be subject to the same mechanism of estrogen action [79].
Other hormonal compounds which have been associated with cancer development include growth hormone and endocrine disruptors. Growth hormone treatment has been found to be associated with an increased risk of central nervous system malignomas in some studies [80]. However, the etiologic role of growth hormone with regard to central nervous system malignomas is controversial, since other studies did not confirm an increased cancer risk among recipients of growth hormone therapy. Several epidemiological, cellular, and molecular studies demonstrate a role of environmental chemicals with endocrine disrupting activities in the pathogenesis of numerous diseases including cancer [81], but their exact role has yet to be defined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree