Fig. 30.1
One- versus two-stage implant-based breast reconstruction
30.3 Specific Techniques
30.3.1 Immediate Implant-Based Reconstruction
Immediate implant-based breast reconstruction after mastectomy is associated with significant psychosocial and quality of life benefits [10]. Four commonly used techniques for immediate implant-based reconstruction are described below: (i) sub-muscular implant placement, (ii) sub-muscular placement with a lower pole sling, (iii) sub-muscular placement with a dermal sling/autograft and (iv) pre-pectoral or subcutaneous implant placement. Whilst the majority of immediate breast reconstructions currently performed are of the two-stage «tissue expander/implant» type (TE/I) [11], all of the techniques described below can also be carried out as a single-stage procedure using either a «direct-to-implant» method or by using an anatomical expandable implant.
Sub-muscular Technique
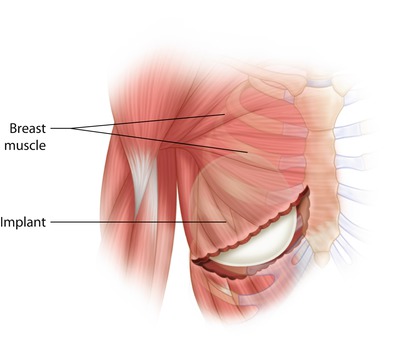
Early implant-based reconstructions involved the placement of implants directly under the mastectomy flap in a «subcutaneous» position. This technique fell out of favour as it was found to be associated with a high degree of complications, as well as unsatisfactory cosmesis. Consequently, techniques were developed in which chest wall musculature was used to provide coverage of the implant, with the aim of reducing implant exposure, palpability and visible rippling by providing an additional soft tissue layer between the incision and the implant. Sub-muscular implant coverage may be «total» (in which sub-pectoral and sub-serratus muscular pockets are created and joined together to provide complete implant coverage) or «partial» (where just a sub-pectoral pocket is created and the lower pole of the implant therefore lies subcutaneously).
- (i)
Total sub-muscular technique (◘ Fig. 30.2)
After completion of the mastectomy and haemostasis, the sub-muscular pocket is prepared. The pectoralis major muscle is elevated from its lateral edge and the sub-muscular pocket dissected medially to the sternal edge. This sub-muscular dissection is continued superiorly to the level of the desired position of the new breast cleavage in the relatively avascular space between pectoralis major and minor. This is usually, but not always, at the level of the second rib. Care should be taken to preserve the large perforator of the second intercostal space because of its significant contribution to the mastectomy flap blood supply. It is important not to extend the pocket too far superiorly as this will result in a high riding implant.
The pectoralis major muscle is elevated from its insertion at the level of the fifth rib to enable implant placement medially. It may be necessary to also elevate part of the anterior rectus fascia, in continuity, to maintain complete coverage. This must be done with care as this layer is often thin and friable.
The lower slips of serratus anterior are elevated in order to provide coverage to the inferolateral portion of the implant. The sub-serratus and sub-pectoral pockets extend down to below the level of the inframammary fold. In a fully sub-muscular technique, if the lower pocket is at the level of the IMF, the implant will appear too high and should be placed approximately 2 cm lower. This is not the case for a partially sub-muscular, dermal sling or mesh supplemented techniques where the IMF is used as the lower border of the implant pocket.
The implant is then inserted into the sub-muscular pocket, and once the position is satisfactory, the lateral border of pectoralis major is sutured to serratus anterior and therefore completely covering the implant with muscle and separating it from the mastectomy space.
The expander (if used) is then expanded to a level which minimises dead space, but without causing tension on the mastectomy or muscle flaps, closed suction drains are inserted inferolaterally, usually with some degree of tunnelling to reduce infection risk, and skin closed over the muscle to complete the procedure.
- (ii)
Partial sub-muscular technique
The pectoralis muscle is elevated as described above but without any further dissection of the chest wall muscles. The implant is placed in the sub-pectoral pocket such that the inferior pole remains subcutaneous when the mastectomy flaps are closed.

Fig. 30.2
Sub-muscular implant placement
Both total and partial sub-muscular techniques are associated with limitations. Firstly since the size of the implant pocket that can be created with either approach is constrained by available skin and muscle, immediate reconstruction generally requires a two-stage (tissue expander/implant) approach unless the patient is very small breasted. With the partial sub-muscular technique, only the superior part of the implant is covered by muscle, which leaves the lower pole of the implant vulnerable to exposure, should skin necrosis or infection occur. In addition, if the flaps are initially thin, or atrophy with time, then the implant and any creases or wrinkles in its surface may be easily palpable. The released lower edge of the pectoralis muscle can also adhere to the skin, such that when it contracts, there is an unsightly deformity known as «window shading» that requires surgical correction. With total muscular coverage, elevation of the serratus anterior muscle can be associated with pain both at the time of surgery and during the expansion phase. Furthermore, tight banding of the raised muscle or fascia across the lower pole may result in difficulty in controlling expansion vectors resulting in flattening and unnatural breast shape. Both techniques can result in lack of control over the position of the IMF, in a flat unnatural look, and make it difficult to achieve a natural looking ptosis.
Sub-muscular and Lower Pole Sling
The «lower pole sling» technique has been developed to avoid the problems associated with partial or total sub-muscular implant placement described above. This technique uses a piece of mesh which is sutured superiorly to the lower border of pectoralis major and inferiorly to the inframammary fold, such that it augments the volume of the implant pocket, lowers the IMF and reduces muscular dissection [12]. Although many different meshes, both biological and synthetic, are now available on the market for this purpose, there is more evidence on the use of «acellular dermal matrices» (ADMs) – as this technique was first described with ADM.
ADMs are sterile, acellular, biological pieces of material derived from human or animal tissue (usually skin), in which the dermis is stripped of cellular components leaving a structurally intact and biochemically inert, extracellular matrix [13]. Whilst the human skin-derived ADM, «Alloderm,» was the first ADM to be described in the literature [14], multiple ADMs have now entered the market derived from both allogenic and xenograft (porcine and bovine) donor sources. As well as dermally derived products, other tissues such as pericardium and peritoneum have also been used to create meshes with similar properties [15]. The first report of using an ADM in reconstructive breast surgery was by Breuing and Warren in 2005, with their series of ten patients who underwent bilateral mastectomy and direct-to-implant single-stage reconstruction using Alloderm [14]. The first series of ADM use in two-stage expander/implant surgery was described by Bindingnavale and colleagues in 2007 [16].
Proposed benefits of ADM use in implant-based reconstruction are described below:
- (i)
Increase rate of direct to implant procedures
The use of ADM to extend the sub-pectoral pocket allows one-stage breast reconstruction to be performed when there is sufficient preservation of the skin at the time of mastectomy. This eliminates the need for the tissue expansion phase. It is suggested that this simplifies the process of reconstruction, potentially reduces the time to completion of reconstruction and therefore minimises cost [17–19].
- (ii)
Reduction in post-operative pain
Reconstructions using ADMs may result in less pain by reducing the extent of muscle elevation to create the implant pocket, thereby reducing the degree of neuronal disruption compared to a standard sub-muscular technique. However the evidence that post-operative pain is reduced by ADM use is purely anecdotal at present. McCarthy and colleagues randomised patients undergoing mastectomy from two US hospitals into cohorts who received two-stage immediate breast reconstruction either with or without ADM and was unable to demonstrate a significant difference in pain scores between the two cohorts either post-operatively or during the expansion phase [20].
- (iii)
Increased fill volumes and fewer expansions resulting in shorter reconstruction time
As a larger pocket may be developed when an ADM is used, it is proposed that this may allow increased initial fill volumes and faster expansions in two-stage procedures. There is conflicting data in the literature with comparative retrospective cohort studies both supporting and refuting this [21]. Factors which may account for this discrepancy include patient factors (the ability of different patients to tolerate varying fill volumes, patient availability and the patient’s physical characteristics) and surgeon preferences for the frequency and volumes of expansions.
- (iv)
Better cosmesis
The rationale behind this theory is that by securing the ADM to the lateral and inferior borders of the breast pocket, this defines the inframammary fold and enables reliable placement of the implant whether placed as one stage or at the time of exchange. Additionally, the alternative technique using complete sub-muscular coverage of tissue expanders restricts the ability of the lower pole to fully expand, whilst placement of an ADM would allow less constriction. This therefore enables full use of the mastectomy flap to allow a natural curve of the lower pole. There are a few studies which report ADM-based reconstructions as having a better cosmetic outcome compared to standard sub-muscular implant placement based on panel assessment of cosmesis from post-operative photographs [22, 23], but follow-up is relatively short.
- (v)
Decreased incidence of capsular contracture
Initial experimental evidence which showed reduced inflammatory reactions associated with ADM use in both animal models and human capsule specimens has been backed up by some clinical studies but has not as yet been evaluated in a prospective trial. Studies with long-term follow-up of ADM-assisted reconstructions have, however, demonstrated a low cumulative rate of capsular contracture even in the setting of radiotherapy [17, 24]. However there are no randomised comparisons, and the rate of capsule formation is still higher in ADM cases when radiotherapy is used so it clearly does not abolish the problem completely [92].
Surgical Technique
Creating the sub-muscular pocket: after mastectomy, the pectoralis major muscle is divided from its origin inferiorly and released medially to the level of 3 (left) or 9 (right) o’clock position. The muscle is then separated from the underlying pectoralis minor and chest wall.
Forming the ADM sling: the ADM is prepared according to the manufacturer’s instructions. Depending on the ADM type, this may require a period of soaking or rehydration. The inferior ADM edge is then sutured to the IMF using interrupted, absorbable sutures. The implant is then put in position in the pectoralis/ADM pocket before the upper border of the ADM is sutured to the lower border of the muscle. Placement of the ADM should be without tension but should not be too loose such as to allow excessive wrinkling or laxity. Extra sutures may be required laterally to fix the ADM to fascia over serratus anterior to define the lateral border and prevent lateral displacement of the implant. When using the inframammary approach and during a subcutaneous mastectomy, it may be easier to suture the ADM to the lower boarder of the pectoralis muscle and then to suture the ADM to the chest wall, after the implant is placed in the pocket (◘ Fig. 30.3).
Drain placement: one (or two) large bore drains may be placed deep or superficial to the ADM to enable adherence of ADM to the skin flap and prevent seroma formation. This is tunnelled and usually removed within 7–10 days. Avoidance of seroma is important in reducing rates of infection and encouraging flap and ADM adherence and integration. Consequently many surgeons leave the drains until drainage rates of less than 30 ml in 24 h are reached.
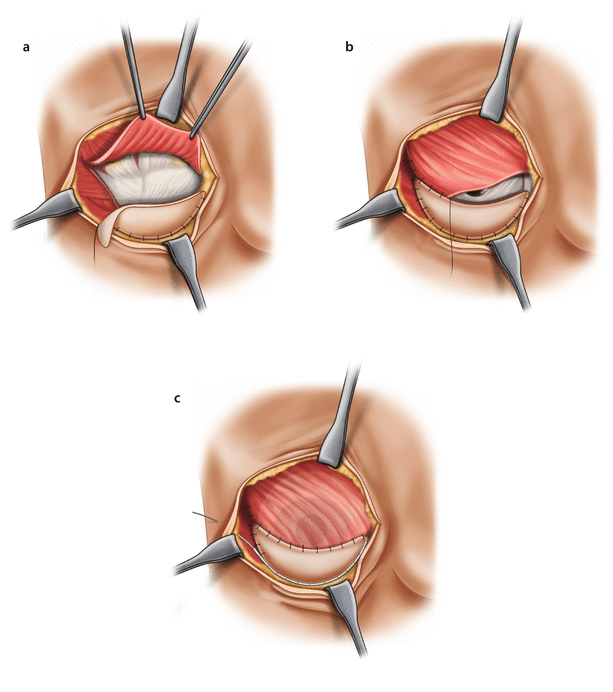
Fig. 30.3
Implant-based reconstruction using an ADM (lower pole sling). a Pectoralis major muscle is raised from the inframammary fold and medially. ADM is sutured to the inframammary fold. b Implant is placed in the newly created pocket and the free upper border of the ADM is sutured to the lower (detached) part of the pectoralis major muscle. c Implant in place within the ADM- muscle pocket
Complications Associated with the Use of Acellular Dermal Matrices
Adverse outcomes associated with ADM use have been the subject of several systematic reviews; some of which have raised concerns that use of ADM may be associated with an increased risk of complications such as infections and seroma formation, when compared with a non-ADM cohort, whilst others report no significant difference [21, 25–28]. The most recent meta-analysis reports that whilst rates of complications such as infection, skin necrosis and seroma are significantly higher, this does not translate into an increased risk of implant loss associated with use of an ADM. It is suggested that these complications are therefore either not serious or that their management has improved such that implant loss is avoided [21]. There is debate, however, as to whether the primary studies used within existing systematic reviews are of sufficient quality to enable such conclusions to be drawn, with the majority being retrospective cohort studies of heterogeneous patient populations, with ill-defined, nonstandardised outcome measures. Potter and colleagues carried out a comprehensive critical appraisal of the evidence base for ADM use in implant-based reconstruction and concluded that the level of evidence from the available primary studies was of such poor quality that combining their results in a meta-analysis was largely inappropriate [29].
Alternative Materials for Lower Pole Support
Widespread acceptance of the lower pole support technique pioneered through the use of ADMs together with a pressure to reduce healthcare costs has led to the development and use of alternative materials for use with the «lower pole sling» technique, both biological and synthetic in nature (◘ Table 30.1). At present comparative studies between products are scarce, and evidence for efficacy is generally based on single centre case series. The considerable cost reduction of using a synthetic material as opposed to an ADM means that if they are found to have equivalent outcomes, they are likely to become an attractive alternative. Furthermore, ADMs are largely available in high-income countries and not available in low- and middle-income countries. Synthetic materials may thus be an attractive option in the latter countries. Use of synthetic meshes has caused some concern due to the difficulty of removal in cases where this is necessary, especially when adherent to a thin skin flap.
Table 30.1
Examples of materials used for lower pole support as an alternative to ADMs
TIGR Matrix | Synthetic mesh | Macroporous mesh made up of two types of copolymer fibres – a fast-degrading fibre which supports the implant during the wound healing phase and a slow-degrading fibre which retains its mechanical properties for 6–9 months. Transient inflammatory reaction only. Cheaper than ADMs |
TiLOOP Bra | Synthetic mesh | Non-absorbable, titanium-coated polypropylene mesh (TCPM) made of a knitted monofilament structure. Available in three different bra-like sizes. Histological studies indicate minimal inflammatory reaction. Cheaper than ADMs |
Vicryl mesh | Synthetic mesh | Dissolves rather than integrates into tissue but no evidence to date that by dissolving rather than integrating, there is a greater risk of implant displacement. One third of the cost of ADMs |
SeriSilk | Biological scaffold | Silk filament combined by helical twisting to form a multifilament fibre which assembled into a three-dimensional scaffold. Behaves like an ADM in vivo as absorption is associated with new tissue generation such that the strength and load-bearing properties are transferred to the newly ingrown tissue. Only a mild inflammatory response |
Sub-muscular and Dermal Sling
The use of a dermal sling in implant-based reconstruction was first described in 1980 by Tanski [30] and further refined by Hammond and colleagues [31], Bostwick [32] and Nava and colleagues [33]. This technique uses a similar principle to the lower pole support offered by ADMs but uses instead the de-epithelialised excessive skin of the lower pole of the breast to provide an entirely autologous, well-vascularised tissue coverage for the lower pole of the implant. This technique is generally offered to women with large ptotic breasts who can be offered a skin-sparing mastectomy, as it makes use of the redundant skin normally excised as part of the skin-reducing Wise pattern incision. Since a skin-reducing incision pattern is used, patients should be counselled that a contralateral mastopexy/reduction is usually required for symmetrisation.
Surgical Technique
Skin flaps are marked preoperatively as per the standard Wise pattern.
Creating the dermal sling: the lower mastectomy flap between and below the vertical limbs is de-epithelialised prior to performing the mastectomy. The de-epithelialised skin is left in continuity with the IMF.
Raising the muscle: the pectoralis major muscle is then raised from its inferior border as described above for the ADM technique. Often, however, there is insufficient dermis to support the implant laterally, in which case the sub-muscular pocket can be extended by continuing the division of the pectoralis origin laterally in a horizontal line, through the fascia and costal digitations of serratus anterior, until the desired pocket width is reached. The sub-serratus pocket is then developed upwards until it joins the sub-pectoral dissection.
Forming the implant pocket: the implant is placed under both the elevated muscle and dermal sling. The upper edge of the dermal sling is sutured to the lower border of the pectoralis muscle to form an implant pocket using interrupted absorbable sutures. Extra sutures may be placed at the IMF or lateral border for better definition.
Drains are inserted in the sub-muscular and subcutaneous spaces, and the horizontal and vertical incisions are closed over the dermal sling implant pocket in the usual fashion.
◘ Figure 30.4 shows a series of photographs of the stages of a dermal sling reconstruction.
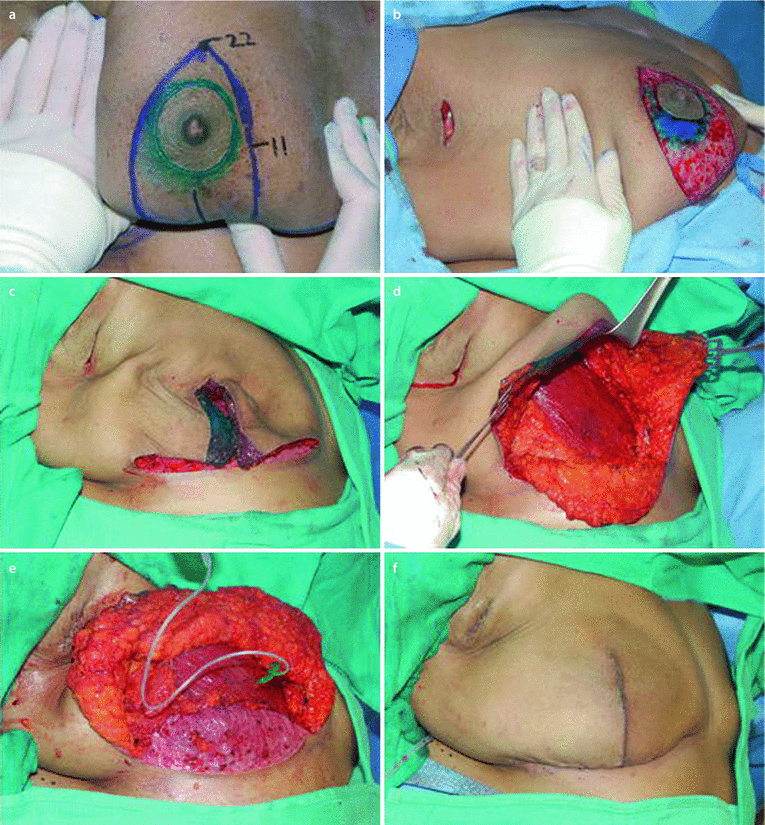
Fig. 30.4
Implant-based reconstruction using a dermal sling (From Dietz [91]. With permission from Springer). a Intraoperative marking showing the apex of the Wise pattern reduction markings at 22cm in this patient. The vertical limbs are 10cm and close to the Nipple-Areola complex (NAC). b Deepithelialization around the NAC prior to hard cut around the nipple and creation of the superior flap. c Deepithelialized superior flap after completion of the mastectomy. d Exposed pectoralis muscle and inferior flap e Pectoralis sewn to inferior autoderm with expansion of the tissue expander. f Final intraoperative view after closure of the flaps
There are only a limited number of case series in the literature reporting on outcomes of the dermal sling technique [34–37]. Whilst earlier case series report high incidences of skin necrosis and implant extrusion, the more recent ones report equivalent outcomes to the use of an ADM [37]. In particular, T-junction dehiscence, which commonly occurs with the Wise pattern incision, is well tolerated due to the underlying dermal sling which prevents implant exposure. Safety and efficacy in the presence of radiotherapy have not been studied to date.
Dermal Sling as an Autograft
A variation on the dermal sling technique makes use of similarly de-epithelialised skin from the excess available when performing a «tummy tuck» procedure and transfers it as a free graft to be used in a similar fashion as an ADM [34–36]. As a result, it is not necessary to use a Wise pattern incision in this technique, but the patient is required to have sufficient excess abdominal skin in order to harvest the graft.
Pre-pectoral or Subcutaneous Implant Place ment
Pre-pectoral implant placement is commonly used for cosmetic breast augmentation but until recently has not generally been used following skin-sparing mastectomy due to unacceptable complication rates, poor cosmetic outcomes and high rates of capsular contracture in early series [93]. With the introduction of biological and synthetic meshes, which provide a replacement for soft tissue coverage, there has been a recent resurgence in this technique. This is based on the rationale that both ADMs and synthetic meshes can be safely placed subcutaneously between the implant and mastectomy flaps when providing support for the inferior pole in retro-pectoral reconstructions described above. There are suggestions that pre-pectoral placement, as well as simplifying the reconstruction overall, may improve patient outcomes by avoiding some of the issues associated with the use of retro-pectoral implant placement such as functional impairment of the pectoralis muscle, breast animation associated with muscle contraction and post-operative pain associated with detachment of pectoralis major as well as during the expander phase in two-stage breast reconstruction. Furthermore by recreating the natural anatomical position of the breast in front of the pectoralis muscle, it is suggested that a more natural appearance may result. Whilst patient selection is generally limited to those with decent subcutaneous tissue coverage, the concomitant use of lipomodelling to improve the quality of the subcutaneous tissue layer may mean this technique becomes available to a larger proportion of women. The technique has several variations, but the general principle involves wrapping either a fixed volume or expander implant within a mesh pocket [38–41]. This pocket may be an acellular dermal matrix or any of the alternatives described above and is either constructed by the surgeon or available in a preformed shape (◘ Fig. 30.5). In the case of expanders, the mesh should be wrapped loosely to allow for an increase in volume. The covered implant is then fixed in position to the underlying chest wall using anchoring sutures between the mesh and the underlying pectoralis muscle. Drains are then placed and skin flaps closed directly over this.
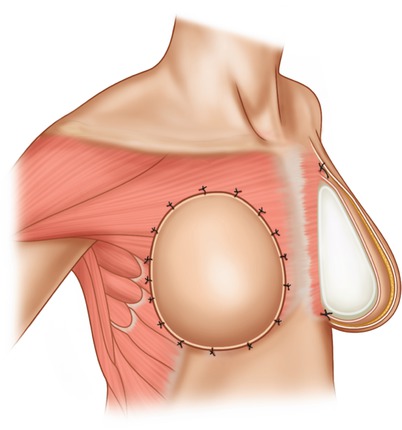

Fig. 30.5
Pre-pectoral implant placement
Although initial outcome data is promising, it is currently too limited at present to make a definitive conclusion as to whether pre-pectoral implant placement offers benefits over the retro-pectoral lower pole sling technique (◘ Table 30.2 presents an overview of studies of pre-pectoral implant placement).
Table 30.2
Published studies on the use of pre-pectoral implant placement
Reference/year | Study type | Mesh used | One stage/two stage | Patients (breasts) | Outcome | Follow-up |
|---|---|---|---|---|---|---|
Reitsamer and Peintinger (2015) [38] | Case series | Strattice (two sheets sutured together) | One stage; fixed volume implant | 13 (22) | No implant loss; no capsular contracture; excellent patient satisfaction and cosmetic outcome | Median 6 months (range 1–12) |
Berna et al. (2017) [40] | Retrospective series (proof of concept) | Braxon© | One-stage; fixed volume | 19 (25) | Three implant losses after first ten patients due to seroma and infection; improved outcomes when mesh to thinner chemical free version. Excellent cosmesis | Median 14 months (range 7–20) |
Bernini et al. (2015) [41] | Non-randomised prospective cohort | TiLOOP (one or two sheets) | One stage; fixed volume implant | Retro-pectoral and TiLOOP: 29 (34) Pre-pectoral and TiLOOP: 30 (35) | Implant loss rate significantly higher in pre-pectoral cohort (5.1% vs 0%) but significantly better cosmetic and patient reported outcomes in pre-pectoral cohort | Retro-pectoral: median 26 months (range 16–42); pre-pectoral 25 months (range 16–40) |
Casella et al. (2014) [39] | Prospective cohort | TiLOOP | Two-stage tissue expander/implant | 25 (25) | No expander or implant losses, infection rate 12% (first stage) and 4% (second stage); no seromas; excellent levels of patient satisfaction with cosmesis | Median 14 months after implant exchange (range 7–23) |
30.3.2 Delayed Implant-Based Breast Reconstruction
A delayed approach may be considered if there are serious concerns about skin flap viability or to minimise the likelihood of complications in high-risk patients where immediate reconstruction would result in unacceptable risk of failure or delay to adjuvant treatment (such as radiotherapy or chemotherapy). Whilst better outcomes are generally associated with an autologous approach, particularly if there has been exposure to radiotherapy [42], implant-based reconstruction may be offered in the delayed setting if patients express a particular preference or if they are considered unsuitable for an autologous technique. It is important that skin quality is assessed, and if it is poor, patients are considered for a latissimus dorsi flap or autologous reconstruction. For implant reconstruction, generally a two-stage tissue expander/implant approach is used as the skin is often too contracted to accommodate a fixed volume implant. The skin at the mastectomy site should be carefully evaluated in terms of its quality and thickness particularly if there has been exposure to radiotherapy, since expansion of thin poor-quality skin is associated with an increased risk of necrotic complications and implant exposure. Another option for irradiated skin is the use of pre-reconstruction autologous fat grafting which has been shown to improve the quality, thickness and vascularity of the skin and subcutaneous tissue resulting in low complication rates and good cosmetic outcomes [43].
The technique used for delayed implant-based reconstruction is similar to the partial or total sub-muscular techniques described above. The mastectomy scar is excised and skin flaps minimally raised just to the extent that the pectoralis muscle is visualised to allow elevation of its lateral border from the underlying chest wall. Further dissection can then proceed in either the subcutaneous plane or sub-muscularly to provide lower pole coverage. Pre-pectoral expander placement could be considered in cases where mastectomy skin flaps are particularly thick and healthy. After pocket dissection, the expander is placed such that maximal expansion occurs in the lower pole in order to produce a more natural breast shape. Intraoperative fill volumes are generally limited due to skin contracture.
30.4 Complications of Implant-Based Reconstruction
30.4.1 Risk Factors for Implant Loss
Most commonly cited complications following implant-based reconstruction are infection, skin necrosis, seroma, haematoma and implant exposure, all of which can result in implant loss if not treated promptly. Even if implant loss does not occur, such complications may have a negative impact on cosmetic outcome. There are several reported risk factors for the development of complications. Whilst there are no absolute contraindications, patients who have such risk factors should be appropriately counselled preoperatively. Identified risk factors which are significantly associated with the development of major complications include smoking, high BMI, older age, large implants or high-volume intraoperative fills of expanders and high ASA scores. Radiotherapy is also associated with increased risk of complications [44–48].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







