Management of Esophago-Gastric Junction (EGJ) cancer with locoregional disease is an area of ongoing discussion and debate, since there is a paucity of randomized trials focusing exclusively on EGJ cancer. Most of the data that guide the multimodality treatment of EGJ adenocarcinoma are derived from trials that involve predominantly gastric or esophageal cancers. Within the trials designed primarily for gastric cancer, patients with EGJ tumors have accounted for only about 20% of all enrollees. There are epidemiologic and pathobiologic differences between EGJ and noncardia gastric adenocarcinomas that raise concern as to whether results from predominantly gastric cancer trials can be extrapolated to EGJ tumors. Similarly, there are histological differences amongst distal esophageal and EGJ tumors. In Siewert’s original description, he recommended that Type 1 EGJ cancers should be treated as esophageal and Type 2 and 3 treated as gastric cancer.1,2 NCCN guidelines based on the seventh edition of the AJCC staging recommends Type 1 and 2 tumors to be staged and treated as esophageal cancer and Type 3 as gastric cancer.3,4 In the following review, we will discuss the available data supporting the operative and multimodality management of EGJ cancers.
In 1998, Stahl et al5 conducted a phase II trial wherein 25 patients with locally advanced (T3-T4 NX M0) squamous cell or adenocarcinoma of the lower esophagus or EGJ were treated with two courses of six weekly administrations of 5-Fluorouracil (2.0 g/m2, 24-hour infusion) and folinic acid (500 mg/m2, 2-hour infusion) combined with twice weekly cisplatin (50 mg/m2, 1-hour infusion). Irradiation of 30 Gy was given concurrently with one course of cisplatin and etoposide. Toxicity was most frequently mild to moderate (WHO grade 1 and 2) with mucositis as the most important side-effect of the preoperative treatment. In total, 94% and 88% completed the chemotherapy and chemoradiotherapy according to the protocol, respectively. Sixteen patients underwent complete resection and 10 of the 16 patients had a complete pathological response. They concluded that an intensive preoperative chemoradiotherapy program is feasible and effective in patients with locally advanced carcinomas of the lower esophagus or EGJ. Although this trial had patients with squamous cell carcinoma of the lower esophagus, it was a precursor study to a more specific evaluation in EGJ adenocarcinoma.
Subsequently, the same investigators conducted a phase III trial6 to investigate whether preoperative combined chemoradiotherapy improves the prognosis compared to chemotherapy alone in patients with locally advanced adenocarcinoma of the EGJ. Patients with locally advanced (uT3-4NXM0) adenocarcinoma of the lower esophagus or gastric cardia were randomly allocated to one of two treatment groups: induction chemotherapy (15 weeks) followed by surgery (arm A); or chemotherapy (12 weeks) followed by chemoradiotherapy (3 weeks) followed by surgery (arm B). Primary outcome was overall survival. A total of 354 patients were needed to detect a 10% increase in 3-year survival from 25% to 35% by the addition of radiation therapy. Unfortunately, only 126 patients were randomly assigned and 119 eligible patients were evaluated. Although the study was closed early due to poor accrual there were several important findings:
Tumors in patients in arm B had a significantly higher probability of showing a complete pathologic response (15.6% vs. 2.0%) or tumor-free lymph nodes (64.4% vs. 37.7%) at resection.
Preoperative radiation therapy improved 3-year survival rate from 27.7% to 47.4% (P = 0.07)
Postoperative mortality was higher in the chemoradiotherapy group (10.2% vs. 3.8%; P = 0.26)
The authors concluded that although statistical significance was not achieved, results point to a possible survival advantage for preoperative chemoradiotherapy compared with preoperative chemotherapy in adenocarcinomas of the EGJ. This is the only trial that is focused exclusively on EGJ tumors and other trials were primarily designed as either gastric or esophageal cancer with varying percentage of patients with EGJ tumors. The key randomized trials that include EGJ tumors are discussed below and are divided into esophageal or gastric trials for the ease of discussion (Tables 94-1 and 94-2).
Selected Randomized Phase III Trials Comparing Neoadjuvant Chemoradiotherapy + Surgery versus Surgery Alonea
| Trial | Number of Patients | EGJ | Histology | Regimen | pCR (%) | Median Survival (Months) | Overall Survival |
|---|---|---|---|---|---|---|---|
| Walsh et al, 19967 | 103 | 39 | 100%AC | Concurrent cisplatin + flourouracil and RT to 40.0 Gy | 25 | 11·0 vs. 16·0 | (3 year) 6% vs. 32%b |
| Urba et al, 20018 | 100 | NA | 75%AC, 25%SCC | Concurrent cisplatin + flourouracil + vinblastine and RT to 45.0 Gy | 28 | 17·6 vs. 16·9 | (3 year) 16% vs. 30% |
| Burmeister et al, 20059 | 256 | NA | 37% SCC, 62% AC, 1% other | Concurrent cisplatin + flourouracil and RT to 35.0 Gy | <16 | 22·2 vs. 19·3 | NA |
| Tepper et al, 200810 | 56 | NA | 75%AC, 25% SCC | Concurrent cisplatin+flourouracil and RT to 50.4 Gy | 40 | 21·5 vs. 53·8 | (5 year) 16% vs. 39%b |
| Van Hagen et al, 201211 | 366 | 88 | 75% AC 23% SCC, 2% other | Concurrent carboplatin and paclitaxel and RT to 41.4 Gy | 29 | 24.0 vs. 49.4 | (5 year) 34% vs. 47%b |
Selected Randomized Phase III Trials Comparing Preoperative/Perioperative Chemotherapy + Surgery versus Surgery Alone for Lower Esophageal, EGJ and Gastric Cancersa
| Trial | No of Patients | EGJ | Regimen | Histology | Median Survival (Months) | Overall Survival |
|---|---|---|---|---|---|---|
| Kelsen et al, 199812 | 440 | NA | Cisplatin + fluorouracil for three cycles before surgery | 204 (46%) SCC, 236 (54%) AC | 14·9 vs. 16·1 | (3 year) 26% vs. 23% |
| MRC, 2002 and Allum et al, 200914 | 802 | 82 | Cisplatin + fluorouracil for two cycles before surgery | 247 (31%) SCC, 533 (66%) AC, 24 (3%) undiff erentiated or unknown | 13·3 vs. 16·8 | (5 year) 17% vs. 23%b |
| Cunningham et al, 200618 | 503 | 58 | Epirubicin + cisplatin + fluorouracil for three cycles before and after surgery | 503 (100%) AC (372 [74%] gastric, 131 [26%] esophageal) | NA | (5 year) 23% vs. 36%b |
| Ychou et al, 201119 | 224 | 144 | 2–3 preoperative cycles of Cisplatin + 5FU and 3–4 cycles postoperatively of same regimen. | 100% AC | NA | (5 year) 24% vs. 38%b |
There has been an interest in trimodality therapy for esophageal cancer for over 25 years in Western countries. Selected trimodality trials will be discussed first (Table 94-1) followed by those trials which incorporate chemotherapy with surgery in the absence of radiotherapy (Table 94-2).
Walsh et al7 conducted a prospective, randomized trial comparing surgery alone with combined chemotherapy, radiotherapy, and surgery in patients with esophageal adenocarcinoma. Patients assigned to multimodal therapy received two courses of chemotherapy in weeks 1 and 6 (fluorouracil, 15 mg per kilogram of body weight daily for five days, and cisplatin, 75 mg per square meter of body-surface area on day 7) and a course of radiotherapy (40 Gy, administered in 15 fractions over a three-week period, beginning concurrently with the first course of chemotherapy), followed by surgery. The patients assigned to surgery had no preoperative therapy. Fifty-eight patients were assigned to multimodal therapy and the 55 assigned to surgery. Key findings include:
Twenty-three of 55 patients (42%) treated with preoperative multimodal therapy had positive nodes or metastases, as compared with 45 of the 55 patients (82%) who underwent surgery alone (p = 0.001).
Tumors in thirteen of the 52 patients (25%) in the multimodal therapy group had complete pathological responses.
The median survival of patients assigned to multimodal therapy was 16 months, as compared with 11 months for those assigned to surgery alone (p = 0.01).
At one, two, and three years, 52%, 37%, and 32%, respectively, of patients assigned to multimodal therapy were alive, as compared with 44%, 26%, and 6% of those assigned to surgery, with the survival advantage favoring multimodal therapy reaching significance at three years (p = 0.01).
The authors concluded that multimodal treatment is superior to surgery alone for patients with resectable adenocarcinoma of the esophagus. This trial was one of the first randomized trials to compare trimodality care to surgery alone for esophageal cancer. It stimulated further trials in this area. However, this trial has been criticized due to the low three year survival in the surgery alone arm (6%).
Based on a pilot study of 43 patients, Urba et al8 designed a randomized trial to compare survival for patients treated with preoperative chemoradiation followed by surgery versus surgery alone. One hundred patients with esophageal carcinoma (25% Squamous cell and 75% adenocarcinoma) were randomized to receive either surgery alone (arm I) or preoperative chemoradiation (arm II) with cisplatin 20 mg/m2/d on days 1 through 5 and 17 through 21, fluorouracil 300 mg/m2/d on days 1 through 21, and vinblastine 1 mg/m2/d on days 1 through 4 and 17 through 20. Radiotherapy consisted of 1.5-Gy fractions twice daily, Monday through Friday over 21 days, to a total dose of 45 Gy. Transhiatal esophagectomy with a cervical esophagogastric anastomosis was performed on approximately day 42. Key findings include:
Median survival was 17.6 months in arm I and 16.9 months in arm II.
Survival at 3 years was 16% in arm I and 30% in arm II (P =.15). At median follow-up of 8.2 years, there was no significant difference in survival between the treatment arms.
This study was statistically powered to detect a relatively large increase in median survival from 1 year to 2.2 years, with at least 80% power and was criticized because the study was underpowered to detect a smaller difference.
Another controlled phase III trial conducted by Burmeister et al9 randomized 256 patients with esophageal cancer including gastric cardia (37% Squamous cell, 62% Adenocarcinoma and 1% others) to surgery alone (n=128) and surgery (n=128) after 80 mg/m2 cisplatin on day 1, 800 mg/m2 fluorouracil on days one to four, with concurrent radiotherapy of 35 Gy given in 15 fractions. The primary endpoint was progression-free survival. Neither progression-free survival nor overall survival differed between the groups, however, the chemoradiotherapy and surgery arm had more complete resections and fewer positive nodes. Major weakness of this study was that only one cycle of chemotherapy was used as was a lower radiation dose (35Gy).
A small Phase III trial (CALGB 9781) conducted by Tepper et al10 enrolled 56 patients with esophageal or EGJ cancers with less than 2 cm spread into gastric cardia, between October 1997 and March 2000. Thirty patients were randomly assigned to trimodality therapy and 26 were assigned to surgery alone. Median follow-up was 6 years. An intent-to-treat analysis showed a median survival of 4.48 v 1.79 years in favor of trimodality therapy (P= .002). Five-year survival was 39% (95% CI, 21% to 57%) vs. 16% (95% CI, 5% to 33%) in favor of trimodality therapy. The trial was closed early because of poor accrual, however it did show a long-term survival advantage with the use of chemoradiotherapy followed by surgery in the treatment of esophageal and EGJ cancers.
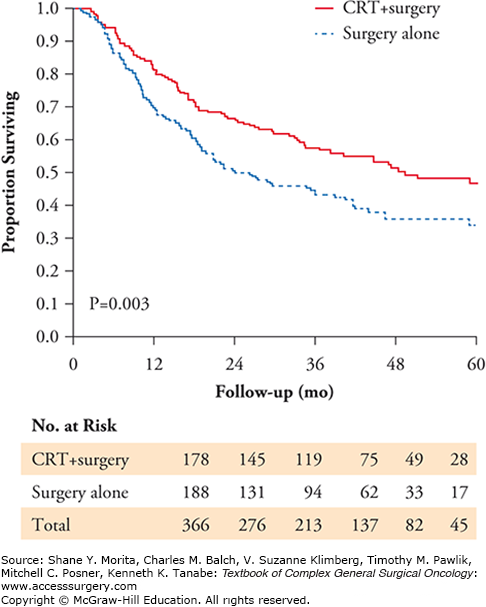
The CROSS trial conducted by Van Hagen et al11 reinforces and solidifies the basis of management of advanced but resectable esophageal and EGJ cancer in the United States and is discussed here in detail. Patients were randomly assigned to receive surgery alone or weekly administration of carboplatin (doses titrated to achieve an area under the curve of 2 mg/ml/minute) and paclitaxel (50 mg/m2 of body-surface area) for 5 weeks and concurrent radiotherapy (41.4 Gy in 23 fractions, 5 days per week), followed by surgery. Of patients enrolled form March 2004 through December 2008, 366 (23% Squamous cell, 75% adenocarcinoma, 2% others) were included in the analysis. Of the 366 patients, 178 were randomly assigned to chemoradiotherapy followed by surgery, and 188 to surgery alone. Eighty eight patients had EGJ tumors. Key findings include:
The most common major hematologic toxic effects in the chemoradiotherapy–surgery group were leukopenia (6%) and neutropenia (2%); the most common major nonhematologic toxic effects were anorexia (5%) and fatigue (3%).
R0 resection was achieved in 92% of patients in the chemoradiotherapy–surgery group versus 69% in the surgery group (p<0.001).
There was a 29% pathological complete response in the chemoradiotherapy–surgery group.
Postoperative complications were similar in the two treatment groups, and in-hospital mortality was 4% in both.
Median overall survival was 49.4 months in the chemoradiotherapy–surgery group versus 24.0 months in the surgery alone group.
Overall survival was significantly better in the chemoradiotherapy–surgery group (hazard ratio, 0.657; 95% confidence interval, 0.495 to 0.871; p = 0.003) (Fig. 94-1).
The authors concluded that since a substantial percentage of patients in the chemoradiotherapy-surgery group had an EGJ tumor, they favor preoperative chemoradiotherapy for these tumors as also suggested by the POET study,6 especially because of the limited toxic effects that were observed with this treatment regimen.
While many western studies have focused on trimodality therapy for esophageal cancer, others have examined the use of preoperative chemotherapy compared to surgery alone (Table 94-2).
The Radiation Therapy Oncology Group trial 8911 (USA Intergroup 113), conducted by Kelsen et al12,13 published their initial and long term results of a randomized trial comparing preoperative chemotherapy followed by surgery vs. surgery alone for esophageal and EGJ cancer. Two hundred sixteen patients received preoperative chemotherapy (Cisplatin and fluorouracil), 227 underwent immediate surgery. There was no difference in overall survival for patients receiving preoperative chemotherapy compared with the surgery only group. They concluded that whether or not preoperative chemotherapy is administered, only an R0 resection results in substantial long-term survival. Due to this negative study in the United States, further studies in the United States focused on the use of chemoradiotherapy as the neoadjuvant treatment approach in esophageal and EGJ cancers.
While negative results were found in the United States, a larger trial in the United Kingdom found different results. Allum et al14 recently published the long term results of a randomized trial conducted by the Medical Research Council Esophageal Cancer Working Party,15 of surgery with or without preoperative chemotherapy in esophageal and EGJ cancer. They recruited 802 patients, 400 in the chemotherapy + surgery (CS) group (two cycles of preoperative cisplatin and fluorouracil followed by surgery) and 402 in the surgery alone (S) group. Results showed that there were 655 deaths, 335 for S and 320 for CS group after a median follow up of 6 years. Five year survival was 23.0% for CS group compared with 17.1% for S group (P=0.03). The authors concluded that preoperative chemotherapy improves survival in operable esophageal cancer and should be considered as a standard of care. The results of this trial have promoted the use of chemotherapy alone as neoadjuvant therapy for esophageal cancer in the United Kingdom. The reasons for the different results in the United Kingdom and the United States regarding the use of neoadjuvant chemotherapy for esophageal cancer is unclear. It is possible that the benefit of the neoadjuvant chemotherapy approach is small and the larger study performed in the United Kingdom was necessary to show its benefit.
McDonald et al16 randomized 556 patients (stage 1b to IVM0 based on 1988 AJCC staging criteria) with resected adenocarcinoma of the stomach or EGJ (108 patients, 20% of the entire study population) to surgery plus postoperative chemoradiotherapy or surgery alone. The adjuvant treatment consisted of fluorouracil plus leucovorin for five days, followed by 4500 cGy of radiation at 180 cGy per day, given 5 days per week for five weeks. One month after the completion of radiotherapy, two five-day cycles of fluorouracil plus leucovorin were given one month apart. Of the 281 patients assigned to the chemoradiotherapy group, 181 (64%) completed treatment as planned. The median overall survival in the surgery only group was 27 months, as compared with 36 months in the chemoradiotherapy group. The hazard ratio for relapse was 1.52 (95% confidence interval, 1.23 to 1.86; p<0.001). Three patients (1%) died from the toxic effects of the chemoradiotherapy; grade 3 toxic effects occurred in 41% and grade 4 toxic effects occurred in 32% of the patients in the chemoradiotherapy group. The authors recommended postoperative chemoradiotherapy for all patients at high risk for recurrence of adenocarcinoma of the stomach or EGJ who have undergone curative resection.
Smalley et al17 recently published their updated results with a 10-year median follow up and demonstrated a strong persistent overall and relapse free survival benefit with adjuvant chemoradiotherapy and recommended this modality for tumors T3 or greater and/or positive nodes. This trial has been criticized for the lack of standardization of the surgery performed by the investigators as well as the toxicity associated with postoperative chemoradiotherapy. A D0 lymph node dissection was done in 54%, D1 in 36% and only 10% of the patients had a D2 lymph node dissection. The debate has been whether postoperative chemoradiotherapy in this trial made up for inadequate lymph node dissection since a D0 dissection entails less than complete dissection of N1 nodes. Nevertheless, this trial illustrates the lack of standardization of surgical care for gastric and EGJ cancers in the United States.
The MAGIC trial conducted by Cunningham et al18 randomly assigned patients with resectable adenocarcinoma of the stomach, EGJ (n=58), or lower esophagus to either perioperative chemotherapy and surgery (250 patients) or surgery alone (253 patients) (Table 94-2). Chemotherapy consisted of three preoperative and three postoperative cycles of intravenous epirubicin and cisplatin on day one, and a continuous intravenous infusion of fluorouracil for 21 days. The primary end point was overall survival.
Rates of postoperative complications and deaths were similar in the perioperative-chemotherapy and surgery group versus the surgery alone group.
The resected tumors were significantly smaller and less advanced in the perioperative-chemotherapy group. There was a significant trend to less advanced nodal disease (i.e., N0 or N1) in the perioperative-chemotherapy group compared to the surgery alone group (Table 94-3).
With a median follow-up of four years, 149 patients in the perioperative-chemotherapy group and 170 in the surgery alone group had died.
Five year overall survival was 36% in the perioperative-chemotherapy group versus 23% in surgery alone group (p=0.009). Similarly progression free survival was significantly better in perioperative-chemotherapy group (p<0.001) (Fig. 94-2).
The results of this trial form the basis of care of gastric cancer in the United Kingdom. In fact, many centers in the United States offer perioperative chemotherapy as per the Magic trial with D2 lymphadenectomy in an effort to avoid the use of postoperative radiotherapy.
The findings of the MAGIC trial18 were supported by another multicenter phase III randomized trial conducted by Ychou et al19 where they randomized patients with resectable adenocarcinoma of the lower esophagus, EGJ (n=144), into either perioperative chemotherapy and surgery (CS group; n = 113) or surgery alone (S group; n = 111, Table 94-2). The primary end point was overall survival. Grade 3 to 4 toxicity occurred in 38% of CS patients (mainly neutropenia) but postoperative morbidity was similar in the two groups. Five-year overall and disease free survival was 38% and 34% in the CS group vs. 24% and 19% (p=0.02 and p=0.003, respectively). Perioperative chemotherapy significantly improved the curative resection rate (84% vs. 73%; p = .04).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree