Skin Cancer: Introduction
Nearly 1.5 million new skin cancers are diagnosed in the United States each year, which represents more than half of all new U.S. cancer diagnoses. Approximately 10 000 people die annually of skin cancer in the United States; one an hour from melanoma and one every 4 hours from nonmelanoma skin cancer. There are three main types of skin cancer: basal cell carcinoma (79% of skin cancers), squamous cell carcinoma (14%), and melanoma (5%). Other skin cancer types comprise the remaining 2%. While melanoma accounts for only 5% of skin cancer diagnoses, it accounts for 75% of skin cancer deaths. Basal and squamous cell carcinomas are considerably less lethal, but these tumors are associated with significant morbidity. As with most malignancies, the incidence of skin cancer increases with age. Owing to their sheer numbers, the public health burden from skin cancer is great.
One in five people in the United States and one in three Caucasians will develop skin cancer during their lifetime. Since 1960, the incidence of skin cancer has risen by 4% to 8% per year. It continues to rise faster than any other cancer, and skin cancer has been labeled as “today’s epidemic” in the lay press. It is likely that much of the data on the incidence and prevalence of skin cancer are an underestimation, as many biopsies of nonmelanoma skin cancer are interpreted in physicians’ offices, and thus go unreported to hospital-based registries.
Etiology
Both genetic and environmental risk factors are implicated in the development of skin cancer. The most common known environmental risk factor is chronic and/or acute intense intermittent ultraviolet light exposure in the form of sunlight, artificial tanning devices (tanning booths), and sunburns. The Centers for Disease Control Behavior Risk Factor Surveillance System data report that nearly 32% of all adults (57% of those aged 18–29) and more than 40% of children in the United States develop at least one sunburn annually. Ultraviolet B (UVB), at wavelengths between 290 to 320 nm, causes most of what we see as sun tanning and sun burns and is responsible for much of the actinic skin damage caused by sunlight. Ultraviolet A (UVA), 320 to 400 nm penetrates more deeply into the skin and is also an important factor in photoaging and skin cancers. UVB radiation induces skin cancer by a variety of mechanisms including direct DNA damage, damage to DNA repair systems, and alteration of the local cutaneous immune system. Some of the strongest evidence that implicates ultraviolet light as being important in the etiology of skin cancer comes from epidemiologic and experimental data correlating the incidence of tumors with the degree of pigmentary protection. Fair-skinned individuals with blue or green eyes and red, blonde, or light brown hair are at highest risk. These patients also typically tan poorly and sunburn and freckle easily. The risk of skin cancer varies with race and ethnic group with Caucasians of Celtic ancestry having the highest incidence rates. Skin cancer is less common in African-Americans, Asians, and Hispanics, but a rise in the Hispanic and Asian population is occurring. Other strong epidemiologic data correlate an increased incidence of skin cancer with residence at lower latitudes where there are higher levels of ambient UVB radiation.
The changes that most persons equate with aging of the skin are a result of chronic solar damage. Prolonged exposure to ultraviolet light leads to cutaneous atrophy, alterations in pigmentation, wrinkling, dryness, telangiectasia, and solar elastosis. Ultraviolet light from the sun or artificial tanning devices is believed to be the most common cause of nonmelanoma skin cancer (NMSC) and may account for some melanomas. Most NMSCs occur on sun-exposed sites such as the face, head, neck, or extensor arms and hands. Understandably, skin cancer is a significant occupational hazard for people who work outdoors. Although NMSCs usually occur on sun-exposed sites, they also rarely occur in sun-protected sites such as the anogenital area, mucous membranes, palms, and soles.
Other environmental/exposure factors associated primarily with squamous cell carcinoma (SCC) include chronic exposure to chemicals such as hydrocarbons (coal tars, soot, cutting oils, and asphalt). Chronic exposure to arsenic is associated with NMSC on both sun-exposed and sun-protected sites. PUVA (psoralen in combination with UVA) used in the treatment of psoriasis and other inflammatory dermatoses produces a dose-dependent increase in SCC and melanoma. Cigarette smoking is linked to SCC of the lip and mouth. The human papilloma virus has been associated with cutaneous SCC in the genital and acral/periungual areas. The carcinogenic effect of ionizing radiation inducing NMSC is documented in human and animal models. Other conditions associated with NMSC include burn and vaccination scars, chronic inflammatory processes and ulcers, and immunosuppression.
A number of genetic syndromes are associated with an increased risk of developing NMSC. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) is an autosomal dominant or mosaic disorder associated with the occurrence of hundreds to thousands of lifetime basal cell carcinomas beginning in childhood or young adulthood. Jaw cysts, palmoplantar pits, abnormal ribs and vertebrae, frontal bossing, and hypertelorism are also found. Albinism, which can occur owing to one of several causal genetic defects, is associated with an increased risk of all skin cancer types. It is characterized by partial or complete failure to produce or distribute ocular, hair, and/or cutaneous UV protective melanin pigment. Xeroderma pigmentosum is a rare autosomal recessive disorder that results in the inability to repair UV-induced DNA damage. It is characterized by hypersensitivity to UV light and the relentless development of skin cancer, especially SCC and melanoma. Individuals with xeroderma pigmentosum are usually diagnosed with their first cancer prior to the age of 10.
A genetic component is also implicated in the pathogenesis of melanoma. Currently, a small percentage of melanomas are known to be associated with chromosomal mutations in CDKN2 A (p16), MC1R, or BRAF. The hereditary nature of melanoma is also noted in families with >100 normal nevi or any number of atypical appearing (dysplastic) nevi. Atypical nevi are not “premelanoma” per se but represent a phenotypic marker for an increased risk of melanoma. A positive family history of melanoma is present in 5% to 15% of melanoma patients. Five to ten percent of individuals with a prior melanoma develop a second melanoma. The genetic etiology of melanoma represents an area of future discovery.
Congenital nevi (CN) are present at birth or appear within the first 6 months of life. The risk of melanoma developing in CN <20 cm in diameter is similar to that of any other area of skin. Melanoma in CN usually occurs after childhood and arises at the dermoepidermal junction making early detection possible. Thus, in the absence of warning signs or symptoms, routine prophylactic removal of CN <20 cm in diameter is rarely indicated. Large (≥20 cm) CN are at increased risk for developing melanoma (5% to 20%), tend to progress to melanoma prior to age 10 (70%), and may originate deep in the lesion resulting in later detection.
Nonmelanoma Skin Cancer
Basal cell carcinoma (BCC) is the most common type of skin cancer and has no known precursor lesion. Approximately 80% of BCCs occur on the head and neck. BCCs are classified by clinical appearance and histologic pattern and include nodular, superficial, aggressive growth, and pigmented subtypes. BCCs rarely metastasize and grow slowly for years prior to sometimes developing a more rapid growth rate. Although they can appear quite indolent, BCCs grow relentlessly and if left untreated can cause extensive local destruction. Occult local invasion can also occur and may be deep and asymmetric with root-like extensions sometimes several centimeters beyond their apparent borders. Metastatic BCC is a rare event (0.03% of all BCCs), most often spreading to the regional lymph nodes followed by the lungs, bones, and skin. The 5-year survival rate for patients with metastatic BCC is poor and patients with distant disease have a median survival of only 10 and 14 months.
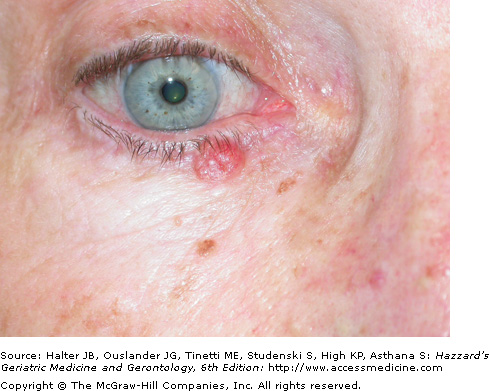
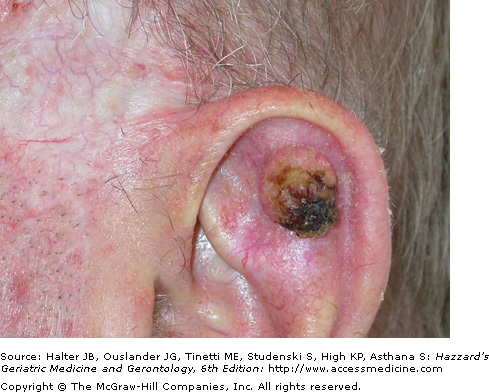
The most common BCC subtype is the well circumscribed nodular or noduloulcerative variant. This typically begins as a small, translucent or waxy flesh-colored or pink, pearly papule or nodule with small telangiectasias on the surface and a translucent, rolled border (Figure 100-1). They may be pruritic or bleed. With time, the nodule increases in size and undergoes central ulceration. Most nodular BCCs present as small (< 1 cm) papules, however, patients still occasionally present with large, disfiguring lesions that have been neglected for many years. Classic histologic features of the nodular BCC include the presence of well circumscribed, basaloid tumor lobules of various sizes in the dermis. The basal cells at the tumor periphery appear to line up, a phenomenon known as peripheral palisading. Areas of separation or retraction artifact between tumor nodules and the adjacent connective tissue embedded in a pale mucinous stroma are also seen.
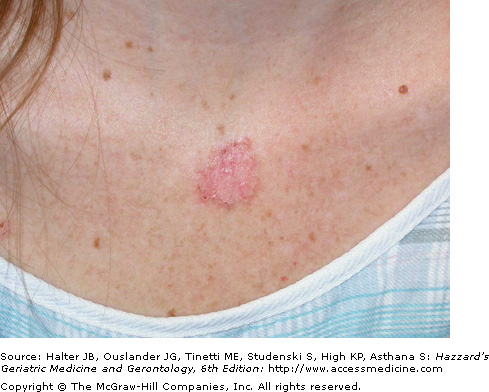
Superficial BCCs occur predominantly on the trunk and usually present as a pink to red, flat scaly lesion, sometimes with a fine, thread-like translucent border with central areas of hypopigmentation or atrophy (Figure 100-2). They are sometimes confused with scaly skin conditions such as psoriasis or other eczematous dermatoses, and may closely resemble actinic keratosis or Bowen disease. Significant and sometimes extensive superficial subclinical involvement is common as they grow by peripheral extension. Histological features of the superficial BCC include numerous, multifocal small buds of basaloid tumor cells that arise in the epidermis and extend into the superficial dermis and down and around hair follicles.
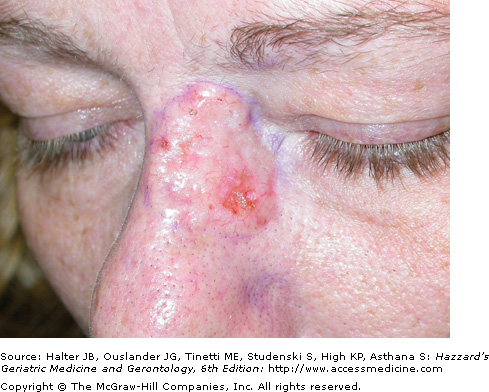
The aggressive BCC subtype usually presents as a flat, firm, indurated, pale, white-to-yellow papule or plaque with harder to detect clinical borders and a “scar like” appearance. This subtype is also known as infiltrating, morpheaform, sclerosing, sclerotic, and fibrotic BCC. Clinically, aggressive BCCs can exhibit subclinical extension widely beyond (>1 cm) their apparent borders, and can also ulcerate (Figure 100-3). They have a higher rate of recurrence following standard treatment modalities because of distant, small finger-like projections of tumor cells that may deeply invade into the dermis, subcutis, and muscle and skate along fascial planes, cartilage, and bone. These small, finger-like islands are often missed with standard histologic margin control. Histological features of aggressive-growth BCC include small islands and spiky finger-like linear strands and cords of basaloid tumor cells embedded in a dense, fibrous sometimes sclerotic connective tissue stroma. Peripheral palisading and stromal retraction is often subtle or absent and perineural invasion is more common. Micronodular BCC similar to aggressive BCC often has significant subclinical extension through the dermis or subcutis. Histologically, smaller tumor islands similar in size to hair bulbs, characterize micronodular BCC. In some cases, a BCC has histologic features of squamous metaplasia and keratinization. These tumors have basosquamous differentiation and may become more aggressive and develop regional lymphatic spread. It is important for the pathologist to convey the BCC histologic subtype pattern to the clinician, especially for aggressive-growth BCC. Multiple histologic patterns may be present in up to 35% to 50% of BCC with the most aggressive pattern correlating with biologic behavior.
Pigmentation from melanin can be seen in all BCC subtypes, but is most commonly seen in nodular BCCs. Some lesions categorized as pigmented BCC are heavily pigmented and can bear a striking resemblance to melanoma or pigmented seborrheic keratoses.
BCC is thought to originate from immature pluripotential epidermal cells. The cytokeratin pattern in BCC resembles that found in the outer root sheath of the hair follicle below the isthmus in the bulge area rather than that of the epidermis, supporting a follicular origin for the tumor. These cells can mature and differentiate in a pattern resembling any of the epithelial structures. BCC can develop in both a hereditary and sporadic fashion. Mutations in the patched (PTCH) gene have been identified in nevoid BCC syndrome and in sporadic BCC. PTCH is a cell membrane receptor for a family of proteins called hedgehog (Hh). The PTCH protein binds and inhibits a transmembrane protein known as smoothened (SMO). Mutations in either PTCH or SMO lead to increased SMO signaling and growth promotion with subsequent cancer formation. The activation of the SMO pathway leads to induction of a number of proteins via the Gli1 transcription factor, including transforming growth factor (TGF-β), platelet-derived growth factor receptor-α, PTCH, and Gli1 and are relevant to cancer development. Mutations that inactivate the tumor suppressor gene p53 have also been identified in more than half of BCCs, but a causal role for p53 mutations in BCC development or progression has not been demonstrated. Patients with Li–Fraumeni syndrome, characterized by an inherited mutation in the p53 gene, have an increased incidence of a number of malignancies, but an increase in NMSC has not been reported.
SCC is the second most common skin cancer type. It possesses the potential to metastasize, the risk of which depends on various risk factors. The most common site of metastasis is the regional lymph nodes, which occurs in 85% of metastatic cases. Approximately 15% of metastases involve distant sites such as the lungs, liver, brain, bone, and skin. The prognosis for patients with metastatic SCC is poor with 10-year survival rates of <20% for patients with advanced regional lymph node involvement and <10% for patients with distant metastases. Fortunately, the majority of cutaneous SCCs are low-risk lesions that are treated with high cure rates.
SCC has a precursor “premalignant” lesion termed actinic keratosis (AK). AKs are extremely common in fair-skinned elderly Caucasian individuals who have had significant chronic sun exposure. AKs usually occur in areas damaged by sun exposure, such as the bald scalp, face, and extensor forearms/hands. AKs occur as well-demarcated, scaly, rough patches or plaques on chronic sun-exposed skin surfaces. Their color can vary from skin colored, erythematous, pink, or to brown, and they are often more easily palpated than seen. The majority of cutaneous SCCs arise in AKs. However, the incidence of progression of an individual lesion from AK to SCC in situ (Bowen disease) and then to invasive SCC remains low, ranging from 0.025% to 20% and occurs slowly over years in immunocompetent individuals. The cumulative risk depends on the number of lesions and the length of time they persist. AKs are easily treatable with cryotherapy or topical agents such as 5-fluorouracil for more numerous lesions. Other conditions predisposing to SCC include radiation exposure; scarring from a previous injury such as a burn, chronic inflammatory processes such as chronic wounds or ulcers, osteomyelitis, sinuses, or discoid lupus; human papilloma virus particularly in acral and genital sites; and chronic arsenic or hydrocarbon exposure.
Patients who are immunosuppressed following organ transplant deserve special consideration and attention. SCC is the most common skin cancer in immunosuppressed organ transplant recipients, occurring 65 to 250 times more frequently than in the general population. Large numbers of premalignant AKs can rapidly develop and progress in fair-skinned, immunosuppressed individuals with a history of significant sun exposure. Moreover, these AKs behave more aggressively and tend to transform into invasive, rapidly growing SCCs with a more aggressive behavior and increased risk of metastasis. Frequent clinical surveillance and early intervention and prevention are thus essential in these patients. Almost half of the malignancies that arise in patients with impaired immune surveillance occur in the skin. The great majority of these are squamous cell cancers, occur in sun-exposed areas, and almost 50% of patients have multiple tumors.
Ultraviolet-induced mutations of the p53 tumor-suppressor gene interfere with apoptosis of the damaged cells, permitting damaged cells to proliferate. More than half of cutaneous SCCs and AKs have p53 gene mutations. Nucleotide sequence analysis demonstrates that these mutations are specifically associated with exposure to UVB light.
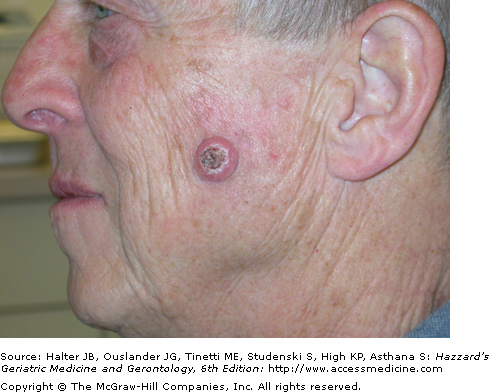
Clinically, cutaneous SCCs most commonly develop (80%) on chronically sun-exposed areas on the head and neck and extremities, followed by the trunk. Clinical presentation varies, but most commonly occurs as a 0.5 to 1.5 cm firm, sometimes tender, hyperkeratotic pink to red papule, nodule, or plaque (Figures 100-4 and 100-5). Ulceration and easy bleeding is a relatively common finding. Rapid and deep local invasion may occur. In darker skinned patients, SCC may present in non sun-exposed areas and is most commonly associated with chronic inflammatory or scarring processes.
Histologically, several subtypes of SCC exist, but the conventional subtype is the most common. Other subtypes include adenoid/acantholytic, bowenoid, spindle/pleomorphic, small cell, verrucous, and keratoacanthoma. The conventional subtype is associated with irregular masses of epidermal cells proliferating downward and invading the dermis. The invading tumor masses are composed of varying proportions of normal squamous cells and atypical cells. These atypical cells demonstrate variations in the size and shape of the cells, hyperchromasia and hyperplasia of the nuclei, absence of intracellular bridges, keratinization of individual cells, and the presence of atypical mitotic figures. In SCC, differentiation is toward keratinization. Horn pearls, which are concentric layers of squamous cells with gradually increasing keratinization toward the center, can also be seen. The dermis often shows a marked inflammatory reaction. Histologic grading of SCC depends on the percentage of keratinizing cells, percentage of atypical cells, number of mitotic figures, and depth of invasion. The Broders classification system is based on the degree of de-differentiation of the cells: in grade 1, <25%; in grade 2, <50%; in grade 3, <75%; and in grade 4, >75% of the cells are undifferentiated (poorly differentiated).
Prior to any treatment, a biopsy confirming the diagnosis is mandatory. Numerous surgical and nonsurgical modalities are effective in treating BCC and SCC. Despite the enormous amount of literature pertaining to the treatment of BCC and SCC, there is relatively little high-level evidence–based research on the efficacy of the treatment modalities used. Most of the reported literature is retrospective, not randomized, and suffers from selection bias, such as allowing treatment modality to be influenced by whether the lesion is low- or high-risk. Based on the best available cumulative evidence, the selection of therapy is based upon several clinical and histologic risk factors noted in Table 100-1. The majority of lesions are relatively low risk and can be treated effectively with standard techniques such as electrodessication and curettage, curettage alone, standard surgical excision, radiation, or ablative cryosurgery with 92% to 98% local control rates for low-risk tumors. As the lesions develop additional risk factors noted in Table 100-1, the control (cure) rate begins to drop precipitously. For higher risk lesions, Mohs surgery or surgical excision with comprehensive margin control or wide margins still results in 95% to 99% local control rates in the majority of cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree