51.1
Introduction
51.1.1
Obesity epidemic
Obesity is a chronic condition characterized by excess weight and body fat, which stems from an interplay between heritable, metabolic, environmental, and behavioral factors. Obesity is defined based on body mass index (BMI) ≥30 kg/m 2 ( Table 51.1 ) and is associated with increased mortality due to its linkage to numerous medical comorbidities such as diabetes, hypertension, dyslipidemia, obstructive sleep apnea, and osteoarthritis. The prevalence of obesity has been gradually increasing since the 1970s throughout the US adult population, reaching nearly 40% in 2015–16 with 8% prevalence of severe/Class III obesity (BMI≥40 kg/m 2 ) . The societal costs of obesity are estimated to be $149 billion in medical expenses per year, which amounts to approximately 9% of annual health-care spending in the United States, and obesity-related medical costs are 42% higher for affected individuals than their healthy-weight counterparts .
| BMI (kg/m 2 ) | ||
|---|---|---|
| Adult | Children and adolescents | |
| Underweight | <18.5 | <5th percentile |
| Normal weight | 18.5–24.9 | 5th to <85th percentile |
| Overweight | 25–29.9 | 85th to <95th percentile |
| Obesity (Class I) | 30–34.9 | ≥95th to 120% of 95th percentile |
| Obesity (Class II) | 35–39.9 | ≥120% to <140% of 95th percentile |
| Extreme obesity (Class III) | ≥40 | ≥140% of 95th percentile |
51.1.2
Role for bariatric surgery
Substantial weight loss, defined as losing >10% of body weight, is associated with improvements in obesity-related conditions but can be difficult to attain using nonsurgical approaches. Among people with severe obesity, an average of 5% weight loss can be achieved through various intensive lifestyle counseling programs with frequent follow-up . With the addition of pharmacologic treatments, there is only an additional 3%–9% weight lost at 1 year . Even when weight loss is realized, weight maintenance can be challenging, in part due to adaptations in metabolism and energy expenditure that favor weight regain.
In contrast, bariatric surgery is a highly effective treatment for severe obesity. An average of 33% of weight or 43 kg is lost , across all bariatric surgical procedures, and is accompanied by marked improvements in obesity-related comorbidities and a reduction in mortality . The long-term weight loss efficacy of bariatric surgery has also been established with 10-year weight loss rates of 14%–25% , accompanied by apparent escape from the inevitable metabolic adaptations that follow conventional weight loss therapies. While these mechanisms are not well understood, bariatric surgery appears to alter the gut and brain in ways that positively impact energy homeostasis and behavior and allow successful weight loss to endure .
Along with dramatic sustained weight loss and a reduction in mortality, bariatric surgery can significantly minimize or reverse obesity-related comorbidities. One metaanalysis demonstrated rates of resolution of diabetes in 92%, hypertension in 75%, obstructive apnea in 96%, as well as an improvement in hyperlipidemia in 76% . Some of these improvements can occur rapidly and take place even before significant weight loss is achieved. For example, insulin sensitivity and secretion has been shown to recover just 1 week after bariatric surgery, leading to improvement of diabetes . The underlying mechanism is not yet known but has been postulated to be related to gut changes and altered secretion of neuroendocrine hormones.
Operative mortality and morbidity overall are relatively low despite the increased surgical risks presented by multiple medical comorbidities in the severe obesity population. For bariatric surgical procedures taken altogether, postoperative mortality is estimated at 0.31%–0.35%, complication rates at 10%–17%, and reoperation rates at 6%–7% . Accordingly, bariatric surgery has risen in popularity worldwide by more than threefold in the past decade. In 2013 more than 460,000 procedures were performed worldwide and approximately 228,000 procedures were done in the United States alone in 2017 .
51.1.3
Indications for bariatric surgery
The eligibility criteria for bariatric surgery in the United States were established by the 1991 National Institutes of Health Consensus Development Conference Panel, with few changes since ( Table 51.2 ). Bariatric surgery should be considered for patients with severe obesity as defined by BMI ≥40 or ≥35 kg/m 2 with one or more obesity-related comorbid conditions . Exclusion criteria include current drug or alcohol abuse; uncontrolled severe psychiatric illness; lack of understanding of risks, benefits, and required lifestyle changes post operation; the presence of reversible disorders as the cause of obesity; and high-risk cardiopulmonary disease. Therefore a multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should perform a careful evaluation of the patient preoperatively and follow with lifelong medical surveillance postoperatively.
| Indications |
| Be well informed and motivated with ability to adhere to postoperative care |
| Have acceptable risk for surgery |
| Have failed previously nonsurgical weight loss management |
| Adults with BMI≥40 |
| Adults with BMI≥35 AND serious comorbidities related to obesity |
| Contraindications |
| Reversible causes of obesity |
| Cardiopulmonary disease making surgical risk prohibitive |
| Current alcohol or drug abuse |
| Lack of capacity |
| Uncontrolled severe psychiatric illness |
In the pediatric population a paucity of data limits evidence-based recommendations for bariatric surgery. The American Academy of Pediatrics recommends surgery be considered for skeletally mature adolescents with BMI ≥50 or ≥40 kg/m 2 in the presence of serious obesity-related comorbidities, whereas the American Society for Metabolic and Bariatric Surgery recommends surgery for children and adolescents with BMI ≥40 kg/m 2 (or 140% of the 95th percentile) or BMI ≥35 kg/m 2 (or 120% of the 95th percentile) with a comorbid condition that might be remedied by durable weight loss .
51.2
Overview of bariatric operations
The concept of bariatric surgery emerged in the 1950s with an initial goal to induce significant and durable weight reduction. It later became increasingly evident that the benefits of bariatric surgery extend beyond weight loss to include various metabolic benefits that are at least partially independent of the weight loss effects. This recognition prompted the bariatric surgery community to introduce the idea of “metabolic surgery” aimed to correct underlying metabolic diseases associated with obesity, such as type 2 diabetes mellitus, hypertension, and hyperlipidemia.
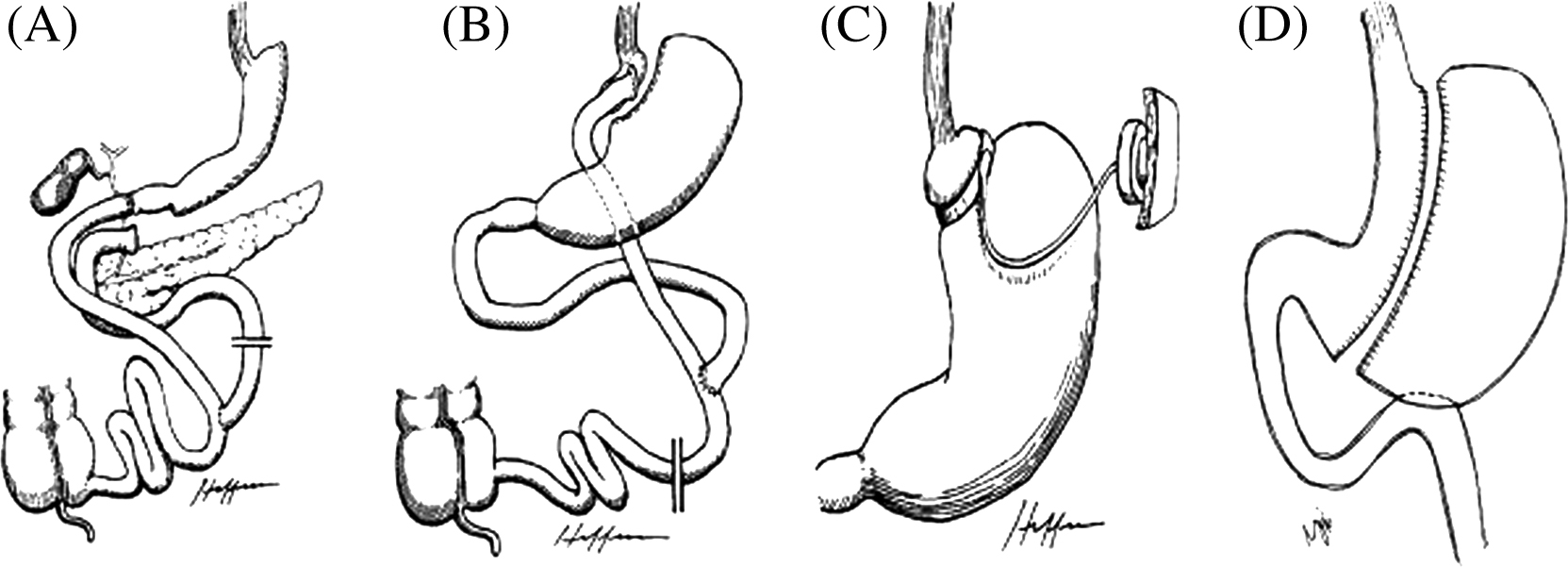
Historically, bariatric surgery has been categorized into restrictive and malabsorptive procedures. Restrictive operations decrease capacity for food intake by reducing the size of the stomach and promoting satiety. Malabsorptive operations reduce the intestinal absorption of calories and nutrients by altering intestinal anatomy and the interaction between bile and pancreatic fluids with ingested nutrients. Some operations combine both components to induce greater weight loss and beneficial metabolic changes in gut hormones and neurohormonal peptides that favor weight loss maintenance. The procedures can be performed open or laparoscopically with increasing preference for laparoscopic procedures given their lower rates of complications and shorter hospital stays. Some of the currently available operations are described in the following sections ( Fig. 51.1 ).
51.2.1
Roux-en-Y gastric bypass
Roux-en-Y gastric bypass (RYGB), sometimes simply called gastric bypass, involves the creation of a small stomach pouch of approximately 15–30 mL in volume that is directly anastomosed to the jejunum, thereby bypassing the greater portion of the stomach, the duodenum, and the proximal jejunum. RYGB is considered a metabolic surgery that has both restrictive and malabsorptive features. For years, it was the most commonly performed operation for weight loss until around 2014 and is still considered the “gold standard” due to its durable weight loss outcomes and associated metabolic benefits.
51.2.2
Sleeve gastrectomy
Vertical sleeve gastrectomy (SG), or SG, involves removing approximately 80% of the stomach and reducing the remaining stomach to a tubular pouch. This procedure is also considered a metabolic surgery and is gaining popularity rapidly given its comparable weight loss result and lower complication rates. It is generally less invasive and less technically challenging than RYGB. The prevalence of SG has increased from 0% to 37% in 10 years worldwide, and it now represents nearly 60% of all bariatric procedures in the United States , making it the most commonly performed bariatric procedure .
51.2.3
Adjustable gastric banding
Adjustable gastric banding (AGB) is a purely restrictive procedure, whereby a band is positioned 1–2 cm below the gastroesophageal junction resulting in a small proximal gastric pouch. The band is connected to a subcutaneously inserted port, and the tightness of the band can be adjusted by injecting sterile saline through the port. AGB is the third most common bariatric surgery performed worldwide, but due to its relatively modest weight loss, risk for late complications and high rates of weight regain , it now accounts only for 3% of all bariatric procedures in the United States .
51.2.4
Biliopancreatic diversion with duodenal switch
Biliopancreatic diversion with duodenal switch (BPD-DS) is a procedure with two parts. The restrictive component is similar to SG with a longitudinal gastrectomy to reduce stomach size by 80%. The intestinal bypass is rendered by transecting and connecting the distal intestine 250 cm from the ileocecal valve to the proximal duodenum 3 cm distal to the pylorus, forming an alimentary limb. Finally, the biliopancreatic limb is attached 100 cm from the ileocecal valve to create a common channel where mixing of biliopancreatic juices occurs. BPD-DS is the fourth most common bariatric surgery performed worldwide, representing 1% of bariatric surgeries that are performed . Although it is one of the most effective procedures for weight loss, the surgery has fallen out of favor given the challenging nature of the operation and higher rates of nutritional complications given the greater degree of malabsorption compared to RYGB.
51.2.5
Other emerging endoscopic procedures
Several endoscopic techniques and devices have become available, although weight loss efficacy and metabolic outcomes remain variable and further studies are needed to better characterize adverse events and longer term complications. Intragastric balloons (IGBs) are generally placed endoscopically in the stomach and filled with saline solution to reduce gastric capacity and promote satiety then retrieved 3–6 months later . Endoscopic sleeve gastroplasty attempts to mimic SG by placing full-thickness stiches endoscopically via mostly vacuum-based suturing systems along the lesser gastric curvature . Duodenal-jejunal bypass liners consist of endoscopic implantation of a 60-cm Teflon tube in the duodenal bulb, allowing food to bypass the entire duodenum and proximal jejunum for 6 months, while gastroduodenojejunal bypass sleeves involve combined endoscopic implantation of a 120-cm tube and laparoscopic approach to fix the proximal end at the GE junction for no longer than 12 months . Aspiration therapy involves insertion of a gastrostomic tube into the stomach, which is then connected to the skin that can be used to evacuate up to 600 mL of each meal about 20 minutes after consumption . Other experimental therapies under investigation include intragastric botulinum toxin injections, electrical stimulation, magnetic compression anastomoses, and duodenal mucosal resurfacing.
51.3
Bone outcomes after bariatric surgery
Initial concerns about skeletal health after bariatric surgery stemmed from observations that there is a higher prevalence of osteoporosis and a greater risk of fracture after gastrectomy . Early bariatric surgical procedures with extensive intestinal bypass and malabsorption, such as jejunoileal bypass and biliopancreatic diversion, were also found to be associated with significant bone loss and histomorphometric changes similar to osteomalacia . These clinical observations have subsequently led to the use of animal models to study the potential negative skeletal consequences of bariatric procedures. Animal models have indeed provided further evidence that surgical manipulation of the gut can adversely affect bone metabolism, with calcium malabsorption, secondary hyperparathyroidism, and progressive bone loss . Furthermore, these deleterious effects on the skeleton are independent of weight loss . Given the health implications, an increasing number of clinical studies have directly examined parameters of skeletal health after more modern and common bariatric procedures.
Clinical application of advanced skeletal imaging modalities has furthered our understanding of the skeletal effects after bariatric surgery. Dual-energy X-ray absorptiometry (DXA) is the modality of choice to assess clinical skeletal health and was the imaging method used in many earlier studies. However, assessment with areal bone mineral density (aBMD) has limitations in the context of significant weight reduction due to soft tissue body composition changes surrounding the bone , leading to concern for artifacts contributing to observed changes. In addition, DXA cannot differentiate between cortical and trabecular bone compartments, evaluate bone microstructures, nor measure bone strength. Therefore more recent studies have employed quantitative computed tomography (QCT) at the axial skeleton and/or high-resolution peripheral quantitative computed tomography (HR-pQCT) to assess volumetric BMD (vBMD), microstructure, and estimated strength at the appendicular skeleton. Although QCT and HR-pQCT may not be immune to the same concerns of obesity-related imaging artifacts, the impact is thought to be less than that for DXA .
This section will focus on human studies and patient-oriented skeletal outcomes after bariatric surgery, organized chronologically from the time of surgery. The body of evidence is most comprehensive for RYGB given that it was the most commonly performed surgery until recently. Less data exist for AGB and BPD-DS as the use of these procedures declined just as interest in studying bone outcomes intensified. Meanwhile, the skeletal effects from SG have not been well characterized given its only recent popularity, and few data are available about the skeletal effects of the endoscopic bariatric procedures.
51.3.1
Short-term outcomes (<6 months after surgery)
51.3.1.1
Clinical changes
For the first 2 weeks after bariatric surgery, patients are started on vitamin/nutritional supplements, placed on a liquid diet, and slowly advanced to pureed and soft foods. Patients generally start to tolerate regular solid foods by 1-month post operation, with a focus on continuing a high-protein diet. Although highly variable between procedures and patients, there is often rapid initial weight loss with an average reduction in absolute BMI by 5–10 kg/m 2 in the initial 6 months following surgery . Type 2 diabetes improves quickly within 7–10 days after surgery with immediate need for medication adjustment, partly owing to restricted caloric intake and increased insulin sensitivity . On average, dyslipidemia improves during the first 6 weeks and hypertension betters in the first week following bariatric surgery .
51.3.1.2
Skeletal changes
Bone turnover makers
There is evidence that increased bone turnover occurs early and dramatically after bariatric surgery . After RYGB, markers of bone resorption go up, with elevations in serum C-terminal telopeptide (CTX) seen as early as 10 days postoperatively . Elevations in urinary deoxypyridinoline (DPD) and urinary/serum N-terminal telopeptide (NTX) of type 1 collagen have been noted at 3 months postoperatively . Meanwhile, the bone formation marker serum osteocalcin (OC) has also been shown to rise at 3 months post-RYGB . Studies comparing SG to RYGB have noted similar increases in biochemical markers in the early postoperative months . BPD-DS also leads to a significant increase in serum CTX as early as 3 days and which continues to increase at 3 months, while serum OC initially decreases at 3 days by 20%, then later increases from baseline at 3 months . On the contrary, the available evidence on AGB indicates that it does not cause an appreciable change in biochemical markers from baseline in the early postoperative period .
51.3.2
Medium-term outcomes (6–12 months after surgery)
51.3.2.1
Clinical changes
By this point, patients are eating a mostly regular diet with continued vitamin and nutritional supplementation. At 1 year following surgery, patients have lost an average of 60% of excess weight and 7–15 kg/m 2 absolute BMI , which is typically the maximum weight loss achieved. RYGB, SG, and BPD-DS are superior with approximately 60%–80% of excess weight lost and an absolute BMI reduction of 10–12 kg/m 2 , as opposed to AGB, which results in 40%–50% of excess weight lost and absolute reduction in BMI of 7 kg/m 2 at 1 year . Other obesity-related comorbidities, including diabetes, dyslipidemia, and OSA, will continue to show improvement corresponding with continuing weight loss. Hypertension, on the other hand, has a high rate of recurrence over time.
51.3.2.2
Skeletal changes
Bone turnover markers
After the immediate postoperative period following RYGB, biochemical markers of bone turnover continue to rise, peaking by 6–12 months . Bone resorption marker serum CTX typically increases by 200% and urinary or serum NTX by 50%–100% during the first year . Bone formation markers also increase but to a lesser extent . This may suggest an underlying mechanism of “uncoupled” bone resorption and formation, which has been described in animal models of RYGB . Elevations in both bone resorption and formation markers are also seen at 1 year after SG, either similar in magnitude or slightly less when comparing to RYGB . Likewise, bone turnover markers continue to rise in the first postoperative year after BPD-DS: serum CTX rises >200%, while OC increases less but still >100% . AGB has variably shown mild-to-moderate increases or no change in bone turnover markers from baseline.
Bone mass
Since the mid-2000s, numerous longitudinal studies have demonstrated marked declines in bone density by DXA and more recently by QCT following RYGB , with changes documented as early as 6 months postoperatively . At the proximal femur, there is a striking decline in aBMD by DXA of 6%–11% within 12 months, which mirrors in magnitude the amount of bone mass loss experienced in the first 3–4 years of menopause. There are now two published studies examining the 12-month decline in proximal femoral vBMD by QCT, both of which revealed less bone mass loss by QCT than when compared with aBMD by DXA . The root cause of these discordant findings between DXA and QCT is not entirely clear. It should be noted that the femoral site showed greater distortion from beam hardening artifact by QCT than the spine QCT imaging ; thus the femoral results should be viewed with caution. It seems likely that bone mass at the hip does decline after RYGB in the first postoperative year, although the exact magnitude remains difficult to ascertain.
At the lumbar spine, changes in aBMD by DXA are more variable across studies but generally decline, although to a lesser extent than the hip . On the other hand, three studies investigating vBMD at the lumbar spine by QCT have shown a consistent decline of impressive magnitude, generally 7%–8% at 12 months . The increased sensitivity of QCT to vertebral bone loss may be explained in part by the propensity of DXA to suffer from artifactually increased bone mass at the spine due to degenerative joint diseases . Overall, there is a decrease in bone mass at the lumbar spine 1 year after RYGB, although DXA may underestimate the drop.
Appendicular skeletal sites, such as the radial bone, have been analyzed as well to determine if nonweight-bearing bones are also affected. Most studies have found declines in aBMD at the ultradistal and total radius but not at the one-third distal radius . Four studies have now evaluated radial and tibial bone density changes with the use of HR-pQCT , showing a small decline in vBMD at the radius and tibia of 1%–2% at 1 year after RYGB. Of note, simulated fat-layering experiments demonstrate that HR-pQCT overestimates vBMD and bone microarchitecture in the setting of overlying adipose tissue , thus leading to an artifactual underestimation of vBMD loss and bone microarchitecture impairment in the setting of decreasing fat mas. The changes observed at both nonweight-bearing and weight-bearing appendicular sites suggest the overall bone loss after RYGB is systemic in nature.
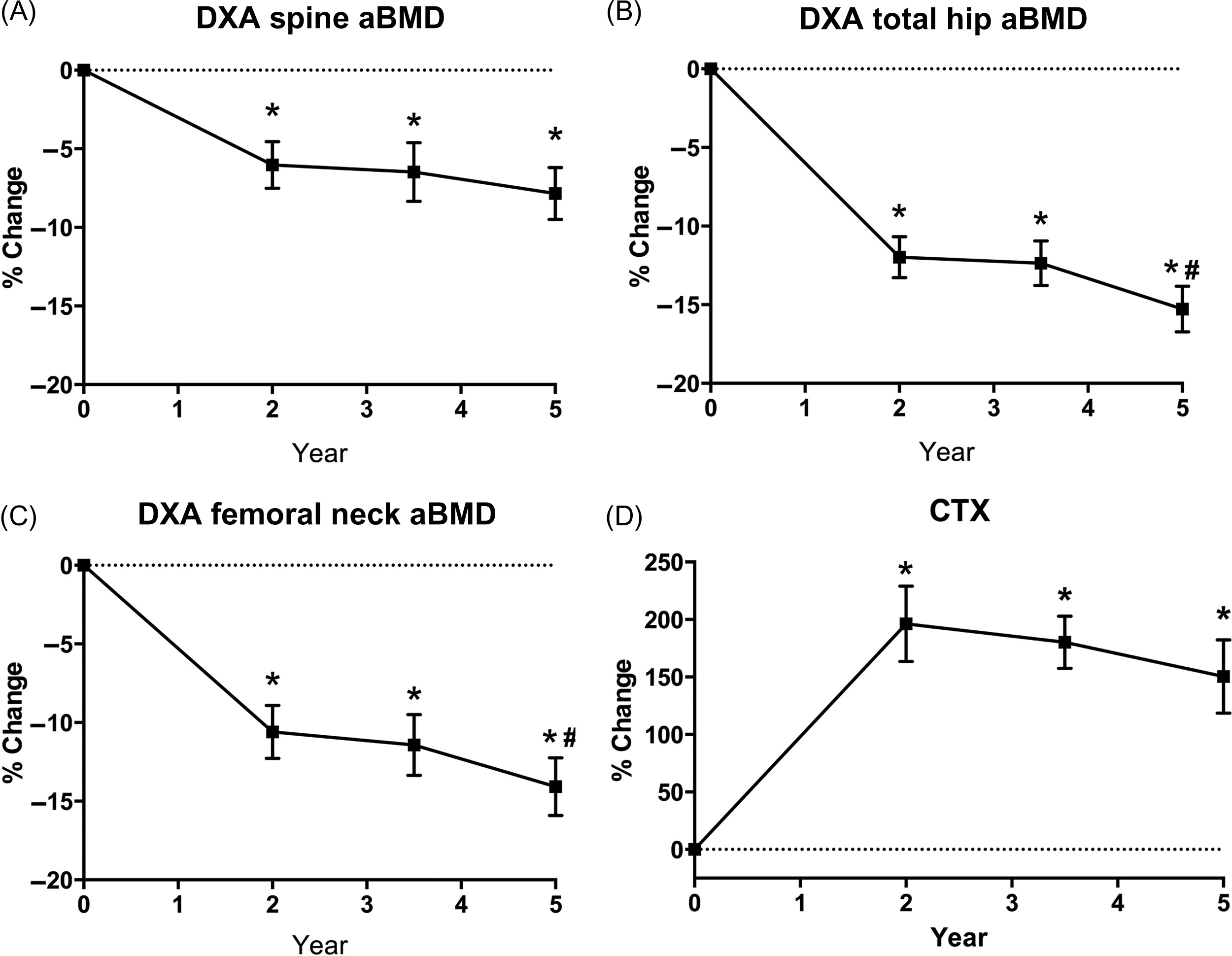
It is clinically important to note that RYGB affects the bone mass of all surgical patient groups, but it has a particularly outsized impact on postmenopausal women, at both axial and appendicular sites ( Fig. 51.2 ). In postmenopausal women, there are not only lower mean DXA-assessed aBMD values postoperatively but also a more marked decline in aBMD by DXA at the hip and lumbar spine as compared to men or premenopausal women . Similarly, the effects of RYGB on bone mass and microstructure at appendicular sites are more significant in postmenopausal women, with a considerable greater decrease in vBMD values and greater increase in cortical porosity and failure load at the tibia .

Far fewer data have examined the skeletal effects after other bariatric procedures, such as SG, BPD-DS, and AGB. SG also appears to lead to bone density declines, but studies are limited by their small sample size, short duration, lack of prospective design, and/or exclusive use of DXA . Based on available data, there appears to be a similar decline in aBMD compared to RYGB. Two studies have used QCT to assess axial vBMD after SG; one showed no change from baseline in vertebral vBMD after 6 months while the other small study showed a 12-month decline in vertebral vBMD comparable or slightly less than those experienced by RYGB patients while there is no change in hip vBMD after SG . After BPD-DS, total aBMD by DXA has been shown to decrease by 10% at 12 months , which is of roughly similar magnitude as RYGB although no direct head-to-head comparisons have been made between BPD-DS and RYGB. After AGB, aBMD by DXA appears to decrease mildly at the hip but notably less than that of RYGB , and no consistent bone density changes are observed at the spine .
Bone microstructure
Only a few studies to date have employed HR-pQCT to quantitatively characterize compartmental microstructure in the first 12 months after bariatric surgery . The majority of these published data are in the post-RYGB population. In the first 12 months after RYGB, there is an overall decline in both trabecular and cortical compartments. At the radius, there is a predominant loss of trabecular bone evidenced by a decrease in trabecular number and an increase in trabecular separation and trabecular network inhomogeneity. The tibia undergoes a decline in both trabecular and cortical bone, with greater trabecular separation and trabecular network inhomogeneity, and increased cortex thinning and cortical porosity , as seen in Fig. 51.2 . There is also an observed reciprocal increase in trabecular area, which is consistent with the process of endocortical resorption.
51.3.3
Long-term outcomes (>12 months after surgery)
51.3.3.1
Clinical changes
Patients’ weights plateau, with some slow regain. The pattern of regain versus weight loss is variable, but the majority of the patients will have a small regain in weight of 4% of baseline weight over several years . Five years after surgery, there is an average 62%–64% excess weight loss with absolute reduction in BMI of 14–15 kg/m 2 after RYGB, 53%–54% excess weight loss with absolute reduction in BMI of 11–12 kg/m 2 after SG, and 47%–48% excess weight loss with absolute reduction in BMI of 9–11 kg/m 2 after AGB . The sustainability of the improvement in obesity-related comorbidities is variable between bariatric procedures: RYGB being more favorable than SG in the long term , and AGB being the least efficacious . Despite reported recurrence of type 2 diabetes in 30%–50% of patients who achieved initial remission after 3–15 years, bariatric surgery is still more effective at long-term control of diabetes when comparing to medical treatment alone , with patients achieving better control with fewer medications. Improvements in other obesity-related comorbidities are mostly stable and sustained.
51.3.3.2
Skeletal changes
Bone turnover markers
Bone turnover markers remain elevated past 12 months after RYGB, although not as pronounced as between 6 and 12 months. Serum CTX continues to be high at 5 years , and urinary DPD and bone alkaline phosphatase are higher at 7 years after surgery than nonsurgical obese controls , despite stabilization of weight. Similar findings of persistently elevated bone turnover markers are found at 24 months after SG , although no longer term data are available. After BPD-DS, marker levels were elevated at 4 years . The data for AGB only extend to 24 months postoperatively, with bone turnover markers variably increased or unchanged from baseline.
Bone mass
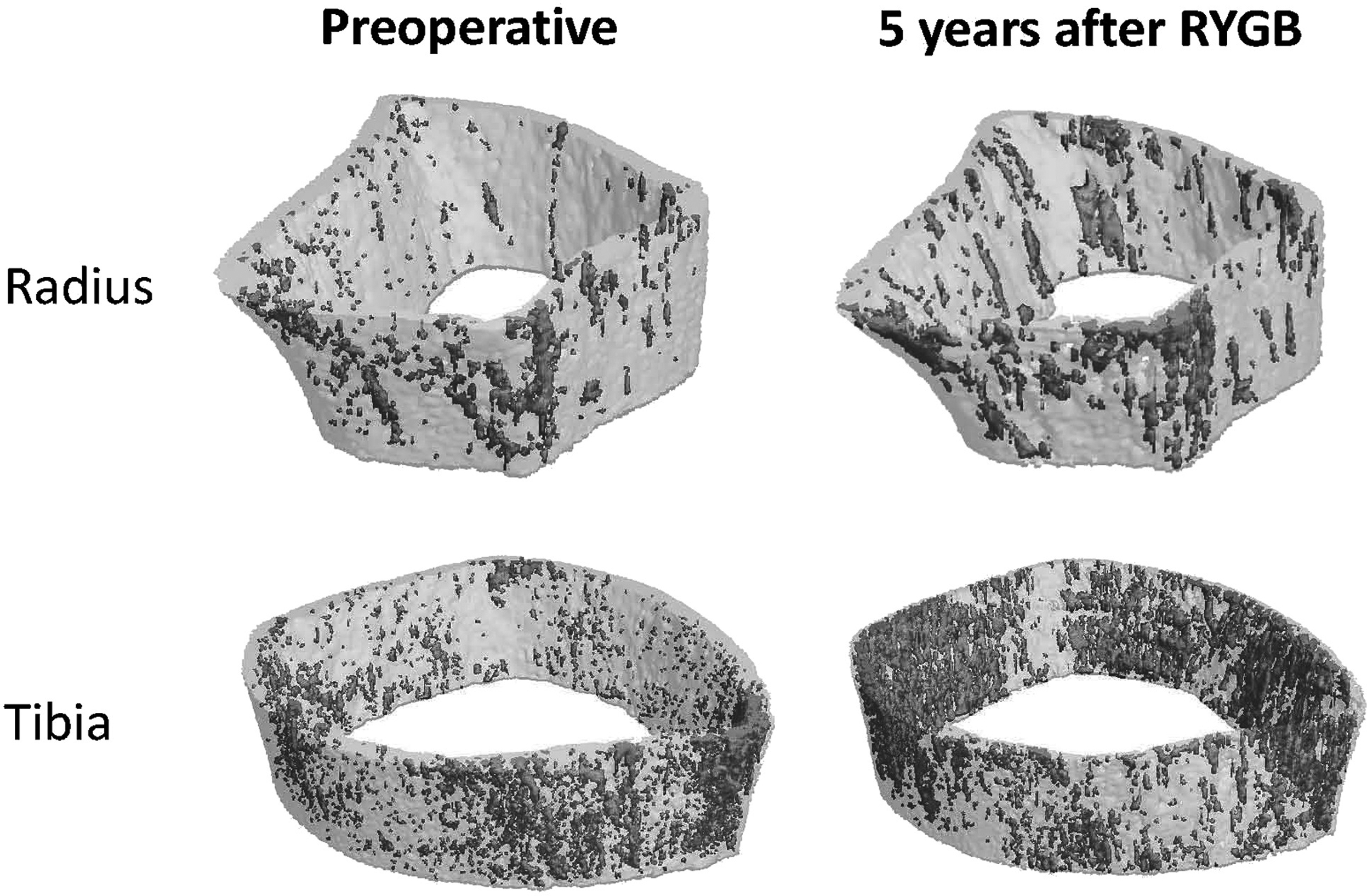
BMD continues to decline over time after RYGB, even after weight plateaus and stabilizes. A progressive decrease in hip aBMD has been demonstrated with RYGB beyond 12 months after surgery. There appears to be greatest bone loss in the first 2 years after surgery, which then continues at a slower rate for up to 5 years based on currently available data . In summary, there is an approximate additional 3% loss in hip aBMD from 1 to 3 years post-RYGB and an additional 1% from 2 to 5 years post-RYGB . One study has evaluated changes in hip vBMD by QCT past 12 months after RYGB, which showed no change at 1 year, but a 7% reduction by 2 years in post-RYGB patients compared with nonsurgical controls, with a resultant similar magnitude of bone loss in the hip assessed by either DXA or QCT . At the lumbar spine a similar progressive loss in bone mass has been observed with a majority of bone loss occurring within the initial 2 years after surgery . Spine BMD shows an additional 3% decline between 1 and 3 years by DXA and 3% decline between 2 and 5 years by QCT . Not enough data are available evaluating aBMD of the appendicular skeleton by DXA past 12 months post-RYGB. However, a few studies have used HR-pQCT to describe the skeletal effects up to 5 years after surgery . Past 1 year after surgery, the decline in total vBMD at the radius and tibia is more apparent with 4%–9% loss by 24 months and 14%–19% by 5 years ( Fig. 51.3 ). This is again consistent with the systemic skeletal effects of RYGB in both weight-bearing and nonweight-bearing sites.
Longer term data for skeletal health after SG are very limited, with the longest follow-up available being 2 years after surgery. Two studies have shown a similar decline in hip aBMD comparing SG to RYGB . Results are more inconsistent with spine aBMD, with one retrospective chart review of 30 SG patients reporting a net increase in aBMD by DXA of 8% at 24 months, whereas a prospective 2-year study demonstrated similar declines at the spine for SG and RYGB . More well-designed and longer term follow-up studies are needed to further understand the skeletal effects after SG, especially with the use of more advanced imaging. After BPD-DS, one small study showed no significant change in aBMD at the hip, but a decrease in spine aBMD by 4% at 10 years after surgery when compared to baseline . Lastly, AGB does not lead to significant bone loss in the long term, with only modest declines in hip aBMD in years 2–3 after surgery and no significant decrease in spine aBMD by DXA .
Bone microstructure
Two studies using HR-pQCT to evaluate bone microstructure after bariatric surgery had follow-up extending up to 24 months and one extending up to 5 years , all including only RYGB patients. The declines in both trabecular and cortical compartments in the first 12 months are matched or exceeded by the decline between 12 and 24 months, with a continual increase in trabecular separation and inhomogeneity and increase in cortical thinning and porosity up to 5 years ( Fig. 51.4 ). The trabecular compartment continues to be the predominant site affected at the radius, whereas both trabecular and cortical compartments are involved at the tibia. Bone strength decreases at both the radius and the tibia based on micro–finite element analysis. The estimated failure load was 9%–10% lower in the radius and tibia by 24 months , and 20% and 13% lower at the radius and tibia, respectively, by 5 years after RYGB . This is consistent with the epidemiologically observed increase in fracture risk after bariatric surgery, described next.
51.3.4
Special consideration in the adolescent population
The observed decline in bone mass after RYGB in adults has raised special concern about the skeletal health of adolescents who have undergone bariatric surgery, as loss of bone density at the age around accrual of peak bone mass could potentially compromise their future bone health. Two studies have evaluated the skeletal changes in this special population. Both showed a baseline of above-normal whole-body BMD Z -score of 1.5–2.0 by DXA with a subsequent significant decrease to Z -score of 0.1–0.5 in the 2 years after RYGB . Although BMD Z-score did not fall below the expected value for gender and age, the dramatic decrease is alarming and longer term data are needed to investigate if bone loss continues at the same rate. The effects of SG or ABG on adolescent bone health have not been studied. After IGB removal, one study found an age-appropriate increase in bone mass at 2 years without indication of a deleterious impact on skeletal health .
51.3.5
Fracture risk
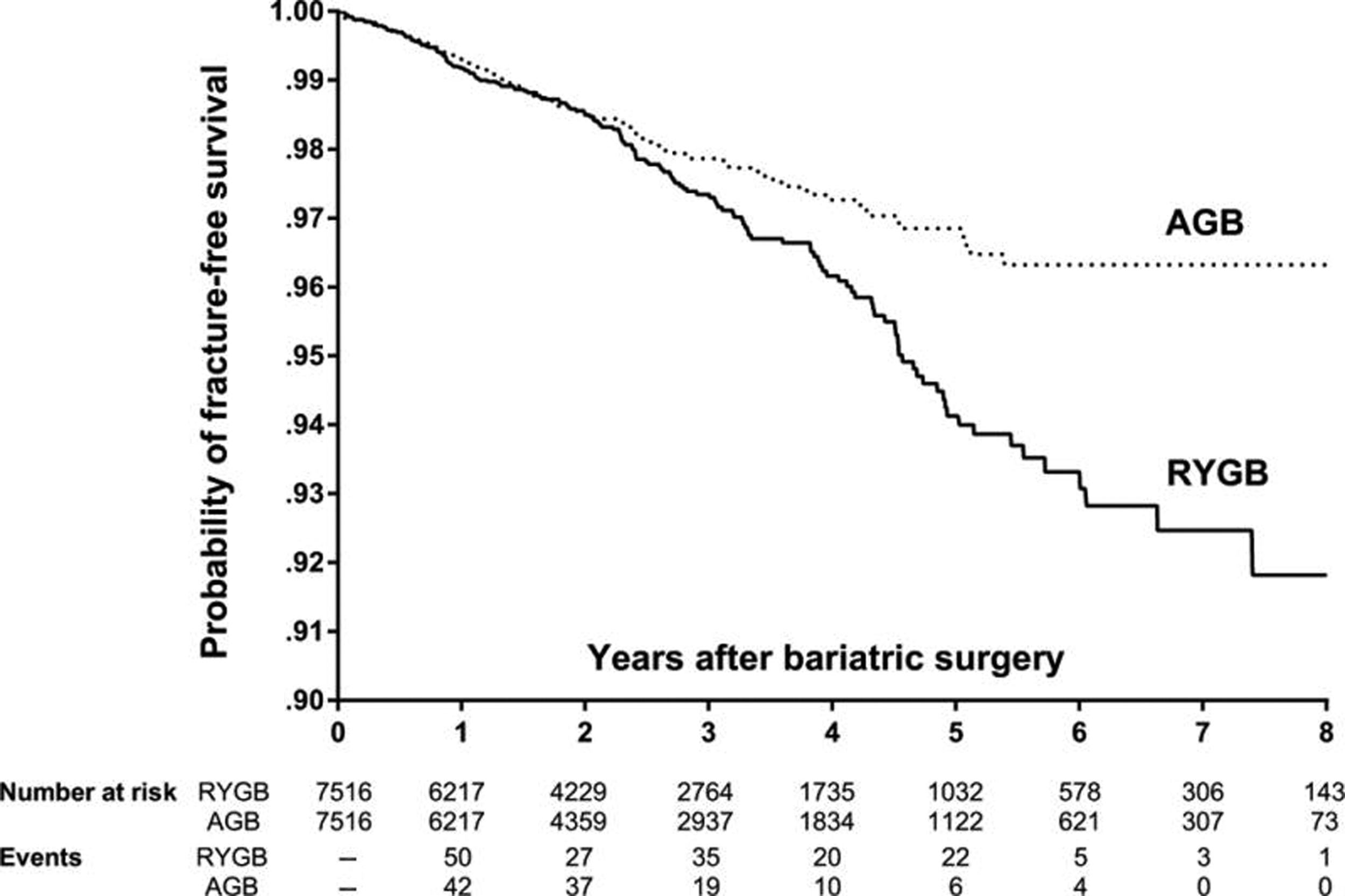
With growing awareness of the observed bone loss after bariatric surgery, several epidemiologic studies provide valuable insight into the impact of various bariatric procedures on fracture risk . First, fracture risk after bariatric surgery appears to vary based on the bariatric procedure. Purely restrictive procedures such as AGB do not appear to be linked to an increased risk of fracture , whereas mixed malabsorptive and restrictive procedures such as RYGB and BPD-DS are associated with a relative risk increase of 1.4–2.3, depending on the study ( Fig. 51.5 ). To date, there are insufficient data to evaluate fracture risk specifically after SG given its relatively recent popularity and thus a lack of long-term data. Overall, postbariatric fractures affect an estimated 10 per 1000 person-years . Thus the absolute fracture risk is low given the relatively young population, but the increased fracture burden in this population as it ages can be worrisome. Second, the fracture risk rises between 2 and 5 years after surgery with an even greater risk past year 5 to 10+ . One study indicates a bimodal distribution of fracture risk, with the first peak occurring at 3 years and the second at year 11 . Although not explored, it is possible that the second peak may have corresponded to the passage of menopause for those premenopausal women undergoing bariatric surgery. Third, fracture risk after bariatric surgery appears to affect osteoporotic sites with an increase fracture events at upper limb (wrist and humerus), spine, hip, or femur .
There are limitations of the currently available evidence, however. The majority of these cohort studies used large population-based databases with varying study designs and marked heterogeneity. Factors, such as the length of follow-up, inclusion of types of bariatric procedures (most included a mixture of different procedures), covariates used to match the control group (most did not match controls by weight or BMI), and adjustment for important confounders, are all differently defined in these studies. In addition, given the relatively low fracture rates in the bariatric group, most studies are not able to assess fracture risk by site. Nevertheless, the available evidence highlights the potential for detrimental bone loss and, therefore, larger long-term population cohort studies matched with controls are needed to further examine the fracture risks.
51.4
Potential mechanisms for postoperative bone changes
Although not fully understood, the negative skeletal consequences of bariatric surgery are presumably multifactorial. Some of the major factors believed to be in play are described in this section.
51.4.1
Nutrition
Of the possible mechanisms of the detrimental skeletal effects of bariatric surgery, nutritional factors are modifiable and therefore create potential opportunities to prevent and treat skeletal complications.
Vitamin D insufficiency or deficiency is highly prevalent in the setting of obesity, so those undergoing bariatric surgery may have suboptimal vitamin D status even preoperatively . After RYGB and BPD-DS, because food and supplements do not mix with pancreatic enzymes and bile until after the biliopancreatic and alimentary limbs converge, vitamin D malabsorption may occur. Moreover, postoperative vitamin D intake may be low, as consumption of food—including vitamin D–containing food—is dramatically restricted . Indeed, insufficient or deficient vitamin D status is highly prevalent after RYGB or BPD-DS . With SG or AGB, preoperative vitamin D insufficiency or deficiency and postoperative dietary intake may still threaten vitamin D status in these patients. Postoperatively, the intestines and biliopancreatic anatomy are intact, and malabsorption of vitamin D should not occur, but vitamin D insufficiency and deficiency are still common . In one patient cohort, for example, vitamin D 1040 IU daily was prescribed (with unreported adherence), but 2 years after SG only 13% of patients had 25-hydroxyvitamin D [25(OH)D] levels at or above 30 ng/mL .
Calcium malabsorption is another potential concern after bariatric surgery. In the gastrointestinal tract, calcium transport occurs through two pathways: an active, transcellular, saturable pathway and a passive, paracellular, nonsaturable pathway. The former predominantly occurs in the duodenum and jejunum, although it does happen in the ileum and colon as well . Paracellular transport occurs throughout the length of the intestine. After RYGB and BPD-DS, one could predict that calcium absorption could decrease meaningfully, due to vitamin D deficiency or the surgical bypass of the duodenum and jejunum. Further, calcium absorption may be impaired by achlorhydria from the bariatric procedure and from postoperative proton pump inhibitor use .
Intestinal fractional calcium absorption has been measured using dual stable isotope methodology in RYGB patients . In a study by Riedt et al., examining 21 women, mean fractional calcium absorption was 36%±8% before RYGB, and at 6 months after surgery had declined to 24%±9% . To what extent vitamin D insufficiency or deficiency played a role was uncertain, as the majority of women had 25(OH)D <25 ng/mL. In addition, calcium intake was <1000 mg postoperatively, on average, and the range of calcium intakes was wide. Schafer et al. hypothesized that if vitamin D status was robust and consistent, and if calcium intake was standardized, these factors could attenuate the negative effect of RYGB on calcium transport. In their study of 33 men and women undergoing RYGB, participants were supplemented to a target 25(OH)D level of ≥30 ng/mL and a 1200 mg/day total calcium intake . Mean fractional calcium absorption was 33%±14% before RYGB, and at 6 months after surgery, it had decreased precipitously to 7%±4% [ Fig. 51.6 ]. As one would expect with a drop in calcium absorption, median parathyroid hormone (PTH) level rose (from 41 to 48 pg/mL, P =.02), 1,25(OH) 2 D level increased, an 24-hour urinary calcium decreased . The fact that the rise in PTH was a modest one might be explained by concomitant non-PTH-mediated stimulation of bone resorption due to other mechanisms; mobilization of calcium from the skeleton would lessen the need for particularly high PTH levels. Larger percentage declines in fractional calcium absorption and lower postoperative calcium absorption were associated with larger increases in the bone resorption marker serum CTX . It is unclear why Riedt and Schafer observed differences in the severity of the FCA decline, but regardless, ensuring robust vitamin D status and calcium intake does not protect a patient from the drop. The more dramatic result of Schafer et al. was reminiscent of early studies of calcium absorption after the jejunoileal bypass, an early weight loss operation .











