Chapter 58 Sexually Transmitted Infections
INTRODUCTION
The importance of the bidirectional relationship between human immunodeficiency virus (HIV)-1 and other sexually transmitted infections (STIs) has become better understood over the past 15 years since the notion of ‘epidemiologic synergy’ between STIs and HIV-1 was first proposed.1,2 Ulcerative and nonulcerative STIs increase the risk of HIV-1 acquisition and transmission and may impact the natural history of HIV-1 infection. Similarly, HIV-1 influences the risk of acquisition, natural history, clinical presentation, and the management of STIs in HIV-infected persons. The multiple and complex epidemiologic and clinical interrelationships between HIV-1 and STIs suggests that effective STI control may be important for HIV-1 prevention.
HIV/STI CO-INFECTION
Diagnosis of HIV in the Setting of STIs
Given the overlap in risks associated with HIV and STIs, diagnosis of an STI should prompt testing for HIV-1, and be used as an opportunity for risk reduction counseling. A particularly severe or atypical presentation of an STI can be a diagnostic clue to HIV-1 infection; neurosyphilis, persistent deep herpetic ulcerations, severe genital warts, and perhaps severe pelvic inflammatory disease (PID) or tuboovarian abscess should increase the clinical suspicion for co-infection with HIV-1. However, most clinical presentations of STIs in HIV-infected persons are no different than among immunocompetent persons and thus typical symptoms and manifestations of STIs should not deter HIV-1 testing. The prevalence and incidence of HIV-1 tends to be higher among sexually transmitted disease (STD) clinic attendees compared to the general population.3–5 In the US, the overall prevalence of HIV-1 in some urban STD clinic settings has been 1–2%, but is even higher in persons with additional HIV risk factors such as injection drug use or men who have sex with men (MSM).4,6–9 Consequently, STD clinics play a key role in the identification of HIV-infected persons and more than a quarter of publicly funded HIV counseling and testing occurs in STD clinics in the US.6 Once HIV is diagnosed, many STD clinics refer patients to HIV specialty clinics, but some STD clinics have begun to provide ongoing HIV care.
STIs and Primary HIV Prevention
Numerous epidemiologic studies have shown that STIs, particularly ulcerative STIs, are associated with increased risk for HIV-1 acquisition.1,2,10–18 Identification and treatment of STIs is considered by most to be an important strategy for the primary prevention of HIV-1. In the community-level randomized trial conducted in Mwanza, Tanzania, improved STI diagnosis and syndromic management reduced HIV-1 incidence by 42% (risk ratio (RR) 0.58; 95% confidence interval (CI), 0.42–0.79; P = 0.007).19 However, two other community-based randomized trials conducted in Rakai and Masaka, Uganda, found that mass treatment for STIs and improved STI management with community education, respectively, did not result in reduced HIV-1 incidence, despite reduced STI rates in the intervention arms.20–22 The contradictory results between these community STI trials may in part be due to differences in the interventions tested as well as the stage of the HIV-1 epidemic with Uganda having a more mature HIV-1 epidemic with higher HIV-1 prevalence (16% and 10% in Rakai and Masaka, respectively), fewer numbers of sexual partners, and lower prevalence of curable STIs compared to Mwanza.1,23–27 Symptomatic STIs may have a more substantial impact on HIV-1 dynamics; in Rakai, symptomatic genital ulcer disease (GUD) (adjusted RR 3.14; 95% CI, 1.98–4.98) and genital discharge or dysuria in men (RR 2.44; 95% CI, 1.17–5.12) were associated with HIV-1 acquisition. However, the majority of incident HIV-1 cases occurred in persons who did not report symptoms and the majority of persons who reported symptoms did not have a curable STI.11 Monthly antibiotic prophylaxis with azithromycin given to female sex workers (FSW) in a randomized trial conducted in Nairobi, Kenya reduced the incidence of STI, but not the incidence of HIV-1 infection.28
In these community-based trials, only curable bacterial STIs were treated and no targeted herpes simplex virus type-2 (HSV-2) interventions were included, even though HSV-2 prevalence was very high in the general population (e.g., 43–47% in women and 13–21% in men) in Mwanza, Rakai, and Masaka26 and 70% in HIV-uninfected FSW in Nairobi.28 In summary, treatment of curable STIs may have played a role in HIV prevention in earlier HIV-1 epidemics, and remains an important priority to reduce adverse reproductive sequelae and burden of STI-associated disease. Recently HSV-2 interventions have become a major focus for HIV-1 prevention.29,30
Diagnosis of STIs in the Setting of HIV
HIV-infected persons should be tested for STIs even in the absence of symptoms, since HIV-1 is associated with high STI prevalence rates in many populations.31–37 Since STIs frequently are asymptomatic, the Centers for Disease Control and Prevention (CDC) recommends screening all HIV-infected persons for STIs at regular intervals and offering treatment based on syndromic diagnoses and laboratory testing.37,38 Detection of an incident STI (e.g., early syphilis or gonorrhea) in a person with known HIV infection may be an indicator of recent high-risk sexual behavior34,39,40 which should prompt risk reduction counseling and, if appropriate and available, additional prevention interventions.
The high incidence of new STI among known HIV-infected persons is concerning.39–44 For example, among 316 HIV-infected women in the US with CD4+ T-lymphocyte cell counts of 300 cells/mm3, 25% were diagnosed with an STI during a median follow-up of 2 years.41 More concerning are data from the Hlabisa district of KwaZulu Natal, South Africa where the prevalence of HIV-1 is 22%, it is estimated that of the 24.9% of women infected with an active STI, 48% are asymptomatic, 50% are symptomatic but do not seek care, and only 1.7% are both symptomatic and seek treatment,45 which highlights why STIs are difficult to control, even in HIV-infected persons.
STIs and Secondary HIV Prevention
The role of STIs in the transmission of HIV-1 has been a public health focus for secondary HIV-1 prevention efforts.40,46,47 Few studies have been conducted to assess predictors of HIV-1 transmission, including STIs, due to logistics of longitudinal HIV-discordant couple studies. The best data come from analysis of monogamous HIV-discordant couples from the Rakai STI trial, which indicated that serum HIV-1 level was the strongest predictor of sexual HIV-1 transmission and that genital ulcers, primarily due to HSV-2, were associated with increased risk of HIV-1 transmission.48 STIs are often associated with increased genital shedding of HIV-149–59 which may correlate with increased HIV-1 infectiousness, although this has not been directly studied. Following appropriate antimicrobial treatment of the specific STI, HIV-1 RNA levels in genital secretions are reduced.49,58,60–64 The effect of STIs on HIV-1 transmission risk is likely greater from males to females.1,15,65 The CDC and the World Health Organization (WHO) promote STI control in persons with known HIV infection as an important component of secondary HIV prevention programs to decrease HIV transmission,38,66 however, successful implementation of STI control efforts have been variable.
GENITAL ULCER DISEASE
Since early in the HIV-1 epidemic, GUD due to syphilis, chancroid, or herpes simplex virus (HSV) and much less commonly due to lymphogranuloma venereum (LGV) and granuloma inguinale, was recognized as a factor for sexual acquisition and transmission of HIV-1 and is thought to have fueled the rapid spread of HIV-1 in sub-Saharan Africa.2,12,65,67,68 Epidemiologic studies have indicated that GUD increases HIV-1 acquisition by two- to sevenfold.11,13–17,69–71 Among the ulcerative STIs, HSV-2 appears to have the strongest and most consistent link to HIV-1 acquisition across continents and populations.10,18 Ulcerative STIs can provide a portal of entry for HIV by disrupting the normal mucosal barrier and cause local inflammation with increased presence of HIV-1 target cells at the site of infection.68,72
Observational studies also have found that GUD increases the likelihood of HIV-1 transmission,73,74 although fewer studies have been conducted to assess predictors of HIV-1 infectiousness. GUD increased the probability of HIV-1 transmission fourfold on a per-contact basis among monogamous HIV-1 discordant heterosexual couples from Rakai, Uganda, after controlling for serum HIV-1 RNA in the index partner.73 HIV-1 has been detected by culture and PCR in genital ulcerations and sometimes in higher levels than in blood.52,53,58,75,76 In a study of women in the Ivory Coast, successful treatment of GUD reduced the amount of genital HIV-1 detected after 1 week to levels similar to women who did not have GUD.53 A high level of HIV-1 RNA in the genital tract is thought to increase the likelihood of HIV-1 transmission, although the threshold for transmission and correlation of genital HIV-1 levels with risk for HIV-1 transmission has not been determined, whereas serum HIV-1 levels have been shown to be a strong predictor of sexual HIV-1 transmission.48
Clinical diagnosis to determine the specific etiology of GUD is often inaccurate even by experienced STI clinicians,77–80 and some patients with genital ulcers may have more than one infection.79–81 In addition, some genital ulcers are caused by trauma or other non-STI etiologies, such as Behçet’s. When possible, the evaluation of all patients with GUD should include syphilis serology, darkfield microscopy, HSV culture, PCR or serology, and culture for Haemophilus ducreyi. However, even after diagnostic testing, at least 25% of persons with GUD lack a laboratory-confirmed etiology79,81 and management should be based on clinical presentation and local epidemiology. In Africa, an increasing proportion of genital ulcers are due to HSV-2 rather than bacterial causes,82,83 resulting in frequent failure of syndromic management of GUD, which usually does not include antiviral therapy.84 The ideal management of GUD in resource-poor countries needs to be re-evaluated, especially in settings with high HIV-1 prevalence, given that HSV-2 is the most common cause of GUD.85
Syphilis–Treponema pallidum
Epidemiology of Syphilis
The recent steep rise in the rates of early syphilis in the United States and Europe is a growing public health concern. With effective treatment available, the implementation of public health measures and the response to acquired immunodeficiency syndrome (AIDS), the rates of early syphilis reached a nadir in 2000 in the US, with most cases occurring in African-Americans living in the Southeast.86,87 Despite efforts to eliminate the disease, syphilis rates in the US and Europe have recently increased, particularly among MSM. Outbreaks of syphilis among MSM have occurred in many major US cities, including Los Angeles, Chicago, New York, Boston, Miami, Seattle, and San Francisco,33,88–94 cities in Europe95–98 and Australia.99 The increased rates of syphilis among MSM are consistent with reports of increased high-risk sexual behavior33,87,100 perhaps due to waning fear of HIV, better health with the advent of effective antiretroviral therapy101 and growing apathy toward safe sex messages.102
A substantial proportion of early syphilis is associated with HIV infection.103–106 In 2002, it was estimated that 25% of primary and secondary syphilis cases in the US occurred in persons infected with HIV-1.104 The overlap of the HIV-1 and syphilis epidemics is particularly marked among MSM.97,104,105,107 In Seattle, Los Angeles, and San Francisco, over 50% of MSM diagnosed with early syphilis were co-infected with HIV-188,91,108 and the odds of HIV-1 infection were from 3.9- to 8.5-fold higher in men with syphilis compared to those without syphilis.103,105,107 Therefore, all persons presenting with syphilis should be offered HIV-1 testing and persons diagnosed with HIV-1 should be screened for syphilis.
Interaction between Syphilis and HIV-1
Laboratory research supports an association between syphilis and HIV-1 acquisition and transmission.109,110 Epidemiologic studies also lend further support for the role of syphilis in HIV-1 acquisition. Among STD clinic attendees in Miami in the early 1990s, patients with primary or secondary syphilis acquired HIV-1 at a rate almost sixfold higher (12.8 vs 2.3 cases per 100 person-years) than patients who never had syphilis and 18% of all HIV-1 seroconversions were attributable to newly acquired syphilis.3 In the past decade, less epidemiologic data have been published that support a relationship between syphilis and HIV-1 acquisition and transmission.
Effect of Syphilis on HIV-1
Syphilis activates the cellular immune system109,111–113 and in vitro has been demonstrated to increase replication of HIV-1.110 One study of 41 co-infected persons found that the treatment of syphilis was associated with an increase in CD4+ T-lymphocyte cells (mean 66 cell/mm3; P = 0.02) and a decrease in HIV-1 RNA (mean −0.261 RNA log10 copies/mL; P = 0.04) compared to pretreatment levels.114 Another study found that plasma HIV-1 RNA levels were higher during primary or secondary syphilis compared with presyphilis levels by a mean of 0.22 RNA log10 copies/mL (P = 0.02).115 However, a retrospective study comparing persons with early syphilis to persons presyphilis and postsyphilis treatment, and controls with nonsystemic STIs (e.g., gonorrhea and chlamydia) found that early syphilis had little effect on plasma HIV-1 RNA levels.116 Treatment of syphilis did not reduce the plasma HIV viral load and had a modest or no effect on CD4+ T-lymphocyte cell counts.115,116 Therefore, it is still debatable whether syphilis has a significant clinical impact on the course of HIV-1 infection.
Clinical Presentation and Natural History of Syphilis in HIV-Infected Persons
Data are conflicting about whether HIV-1 infection affects the clinical manifestation of syphilis. There have been several case reports of aggressive or atypical syphilis in HIV-infected persons,117–122 but larger controlled trials have observed only minor or no significant differences in the clinical manifestations of early syphilis between HIV-1-infected and HIV-1-uninfected persons.123–125 In a prospective, randomized, multicenter study of early syphilis in the US, the median number of ulcers and percentage of persons with multiple ulcers having primary syphilis was greater among the 53 HIV-infected persons. However, no other differences in clinical manifestations were noted in comparison to the 200 HIV-negative controls.125 A study of 677 men from Malawi showed that HIV-infected persons had impaired healing of genital ulcers (P = 0.003), of which 29% were due to syphilis.126
Several studies suggested more rapid progression of syphilis in HIV-infected persons since they more often have secondary syphilis at the time of presentation and occasionally have concomitant manifestations of primary and secondary syphilis.127–131 In a case-control study, 53% of patients with HIV-1 presented with secondary syphilis compared to 33% of patients without HIV-1 infection (P = 0.01).128 In the US prospective study referenced above, 25% of HIV-infected persons with secondary syphilis presented with concomitant chancres compared with only 14% of HIV-uninfected persons.125 It was not possible to determine whether the HIV-infected persons truly progressed more rapidly to secondary stage, had delayed healing of primary chancres, or sought healthcare earlier. In contrast to these results, Gourevitch and co-workers did not find that the stage of syphilis at the time of presentation was associated with HIV status.123
Diagnosis of Syphilis in HIV-Infected Persons
Most experts believe that syphilis serologies are accurate and reliable in the vast majority of HIV-infected individuals and can be used for the diagnosis of syphilis and for monitoring the response to treatment in the usual manner.66,125,132–135 Nonetheless, occasionally HIV-infected persons can have atypical syphilis serologic results, with higher titers or more biologic false-positives.123,136–140 The higher rate of biologic false-positive rapid plasma reagin (RPR) may be due to injection drug use, rather than HIV infection itself.133,138 Infrequently, lower titers, delayed seroreactivity, and false-negatives have been reported in primary, and less commonly, in secondary syphilis.141–144 When syphilis serology and clinical presentation do not correspond, other diagnostic tests for syphilis should be used, such as direct microscopy with darkfield examination or fluorescent antibody staining of exudate, or in difficult diagnostic cases, biopsy.66,143
Neurosyphilis
T. pallidum invades the central nervous system early in the course of disease, but only persists in a subset of persons who are at higher risk for developing neurosyphilis. Unfortunately, it is impossible to predict which patients are at greater risk for persistence. Some studies suggest that HIV-infected persons have higher likelihood of neurosyphilis, including symptomatic and asymptomatic meningitis, ocular disease, and meningovasculitis.145–148 In the absence of neurologic symptoms, 9–58% of HIV-infected persons with serologic evidence of syphilis have neurologic involvement.128,149–151 In a recent study of 326 persons with early and late syphilis, 72% of whom were HIV-infected, those with serum RPR titers of ≥1:32 and CD4+ T-lymphocyte cell counts of ≤350 cells/mm3 were more likely to have laboratory-defined neurosyphilis.152
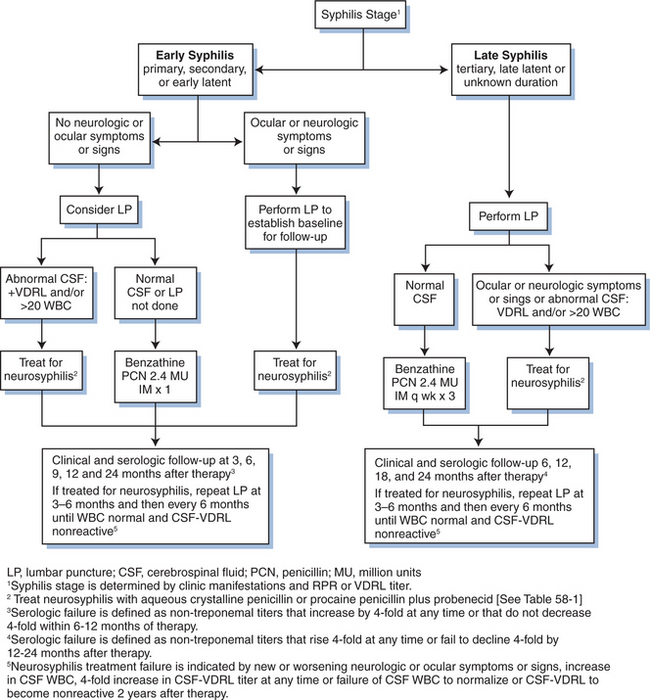
At any syphilis stage, if neurologic symptoms are present, persons should be treated for neurosyphilis. Examination of the CSF prior to treatment of neurosyphilis establishes a baseline for later evaluation of the CSF to determine response to treatment (Fig. 58-1). Some experts recommend CSF examination before treatment of HIV-infected persons with early syphilis regardless of neurologic signs or symptoms, because HIV-infected patients with early syphilis may be at higher risk for developing neurosyphilis after appropriate treat-ment.129–131,159–164 If CSF results are abnormal then treatment for neurosyphilis should be initiated (Fig. 58-1 and Table 58-1). Most experts agree that all HIV-infected persons with late latent syphilis, tertiary syphilis, or syphilis of unknown duration should undergo a lumbar puncture and treatment decisions should be based on the CSF examination (Fig. 58-1).66
A reactive CSF-VDRL is considered diagnostic of neurosyphilis, but can be falsely negative in up to 70% of neurosyphilis cases.153 Thus, the diagnosis may need to be based on CSF pleocytosis or elevated protein concentration. Because CSF abnormalities, including mild pleocytosis and elevated protein levels, are common in patients with HIV infection alone,154,155 the clinical significance of such CSF abnormalities in HIV-infected persons with syphilis is often difficult to interpret. However, many experts recommend that HIV-infected persons with syphilis who have a CSF white blood cell count above 20 cells/μL be treated for neurosyphilis, even if the CSF-VDRL is nonreactive. The CSF fluorescent treponemal antibody (FTA) and FTA-absorbed (FTA-ABS) tests are more sensitive, although less specific for neurosyphilis, and can be used to help rule out the diagnosis of neurosyphilis when the CSF-VDRL is nonreactive.156–158
Treatment of Syphilis and Serologic Response to Therapy in HIV-Infected Persons
Early Syphilis (Primary, Secondary, or Early Latent)
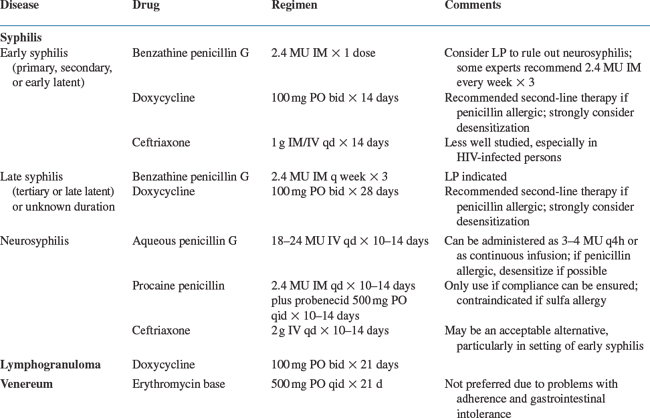
Most HIV-1-infected persons with early syphilis respond to standard benzathine penicillin G (BPG) therapy as is recommended for HIV-1-uninfected persons.123,124 Some experts choose to treat HIV-1-infected persons with early syphilis more aggressively, with 2.4 million units weekly for 3 weeks, due to concerns that the recommended single injection of 2.4 million units of BPG may not be adequate,66,131 although there is no evidence that three weekly doses are any more effective than standard therapy for early syphilis.
Given the higher risk for treatment failure and neurologic involvement among HIV-1-infected persons,129,160,165 careful follow-up is essential. HIV-1-infected patients with early syphilis should be clinically and serologically evaluated for treatment response at 3, 6, 9, 12, and 24 months after therapy.66 As with HIV-1-uninfected patients, HIV-1-infected persons with clinical treatment failure or titers that do not decrease fourfold within 6–12 months after therapy, should undergo CSF examination and re-treatment as indicated by CSF findings (Fig. 58-1). If the CSF is normal, most experts would treat with intramuscular BPG 2.4 million units once a week for 3 weeks; whereas, abnormal CSF should prompt treatment for neurosyphilis.66 The management of penicillin allergy is the same for HIV-1-infected and HIV-1-uninfected patients.
Alternatives to penicillin have not been well studied in HIV-1-infected persons and should be used with caution. The CDC recommends doxycycline 100 mg twice a day for 14 days as second-line treatment for early syphilis if a person is penicillin allergic or refuses a parenteral regimen.66 A retrospective study comparing BPG to doxycycline for the treatment of persons with early syphilis, a minority of whom were HIV-infected, found no difference in the time to serologic response.166 In a small study of HIV-1-infected persons, the serologic response rates for persons with early syphilis treated with BPG and doxycycline were also similar.114 Other options for treating early syphilis include 1 g of intramuscular or intravenous ceftriaxone daily for 10 days. A single oral 2 g dose of azithromycin had been a promising alternative treatment, but treatment failure and identification of azithromycin-resistant isolates indicate that azithromycin should not be used for the treatment of syphilis.167–169
Most studies suggest that the serological response after treatment for early syphilis is no different in HIV-1-infected and HIV-1-uninfected persons. A study in Baltimore showed a similar rate of decline in RPR titer during the 12-month period after standard treatment for early syphilis among HIV-1-infected and HIV-1-uninfected patients and baseline serum RPR titer and CD4+ T-lymphocyte cell count did not predict clinical failure.128 Similarly, a case-control study in New York City found no difference in serological response 6 months after treatment for secondary syphilis in HIV-1-infected and HIV-1-uninfected persons, though the HIV-1-infected persons with primary syphilis were less likely to have at least a fourfold decrease in RPR titers.170
Late Syphilis (Late Latent Syphilis, Tertiary Syphilis) or Syphilis of Unknown Duration
HIV-1-infected persons with tertiary syphilis, late latent syphilis or syphilis of unknown duration without evidence of neurosyphilis should be treated with 2.4 million units BPG intramuscularly once a week for 3 weeks and then clinically and serologically evaluated at 6, 12, 18, and 24 months after therapy.66 If at any time neurological symptoms develop, nontreponemal titers rise fourfold, or if in 12–24 months the nontreponemal titer does not decline fourfold, CSF examination should be repeated and treatment further determined based on CSF findings. Because nonpenicillin regimens have not been well investigated in HIV-infected persons, these should be used with caution. The management of penicillin allergy is the same for HIV-1-infected and HIV-1-uninfected patients; desensitize to penicillin if possible. If indicated, doxycycline 100 mg twice daily for 28 days is an alternative regimen, but compliance should be closely monitored.66
The serological response after treatment for late syphilis is no different in HIV-1-infected and HIV-1-uninfected persons. Among women in Zaire treated for late syphilis, the percentage of HIV-1-infected women with at least a fourfold decline in the RPR titer by 2 years was similar to HIV-uninfected women, regardless of the baseline RPR titer.171
Neurosyphilis
Regardless of the stage of disease, if CSF abnormalities consistent with neurosyphilis are found (reactive CSF-VDRL or elevated CSF WBC), the patient should be treated for neurosyphilis with 18–24 million units of aqueous penicillin G intravenously divided into 3–4 million units every 4 h or as a continuous infusion for 10–14 days (Fig. 58-1 and Table 58-1). If compliance can be ensured, 2.4 million units of procaine penicillin intramuscularly can be given daily along with probenecid 500 mg orally four times per day for 10–14 days as an easier outpatient alternative. Ceftriaxone may also be an acceptable alternative to penicillin in HIV-1-infected persons with neurosyphilis in early disease.66
After neurosyphilis treatment, CSF should be reexamined after 3–6 months and then every 6 months thereafter until the abnormalities resolve; normalization of CSF protein in HIV-1-infected patients often does not occur. Failure of protein to normalize should not prompt re-treatment.172 Resolution of CSF abnormalities may be slower after neurosyphilis treatment in HIV-1-infected patients.172,173 In one study, HIV-1-infected persons were 2.5 times less likely to revert to a nonreactive CSF-VDRL compared to HIV-1-uninfected persons, and 3.7 times less likely if the CD4+ T-lymphocyte cell count was ≤200 cells/mm3.172 Another study found that at 24 weeks after treatment, although the CSF-VDRL titer decreased or reverted to nonreactive in only four of 11 persons with neurosyphilis, T. pallidum was not detected by PCR or isolated by rabbit-infectivity testing in any CSF specimens after treatment.164 Despite clinical resolution of symptoms, two participants had no improvement in any of the tested parameters, including serum RPR titer, CSF-VDRL titer, CSF white cell count and CSF protein concentration, and one person relapsed, despite high-dose penicillin therapy.
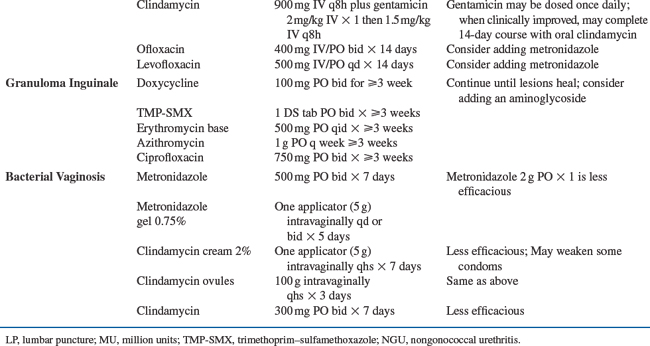
Chancroid–Haemophilus ducreyi
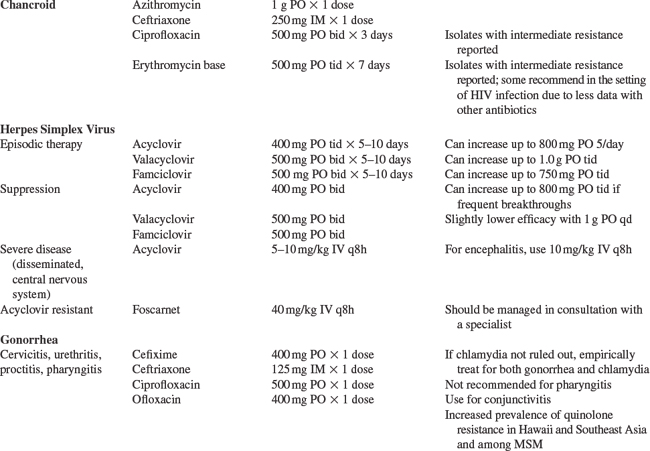
The prevalence of chancroid is decreasing worldwide and HSV-2 is now a much more common cause of GUD than chancroid.85,174–177 In the 1980s and early 1990s, chancroid was a cofactor for HIV acquisition and transmission, when the prevalence of chancroid was high in sub-Saharan Africa.178–180 The clinical presentation of chancroid in HIV-1-infected persons is similar to HIV-1-uninfected persons, although there have been reports of atypical presentations, longer incubation period, slower ulcer healing, and greater number of ulcers in the setting of HIV-1 infection.181–183 The management of chancroid in HIV-1-infected patients is the same as HIV-1-uninfected persons (Table 58-1), however, occasionally HIV-1-infected patients may require a longer treatment course and treatment failure has been reported.184 Most persons with chancroid are treated syndromically, since the bulk of disease occurs in the developing world where chancroid culture and PCR are rarely available.
LGV–Chlamydia trachomatis Serovars L1–L3
Epidemiology of LGV and HIV-1
LGV, a previously rare STI in the Western world, has had a recent resurgence. There have been several outbreaks of anorectal LGV among MSM in Europe and the US and 57–87% of the cases were found to be co-infected with HIV-1.185–190 In Amsterdam, HIV seropositivity was found to be the strongest risk factor for LGV infection compared to MSM without any chlamydial infection (OR, 9.3; 95% CI, 4.4–20.0).191 Many LGV cases had several other concomitant STIs diagnosed and reported high-risk sexual behavior.186,189
Clinical Presentation, Diagnosis, and Treatment of LGV in Persons with HIV-1 Infection
LGV is an STI caused by Chlamydia trachomatis (CT) serovars L1–L3 that classically presents with inguinal buboes associated with a genital ulcer or papule. Among MSM, LGV often presents with rectal manifestations ranging from mild constipation and rectal discharge, perianal ulcerations, to severe fistulous proctitis resulting in permanent scarring. During an outbreak in the Netherlands, ulcerative proctitis was visualized in all nine men who underwent sigmoidoscopy.186
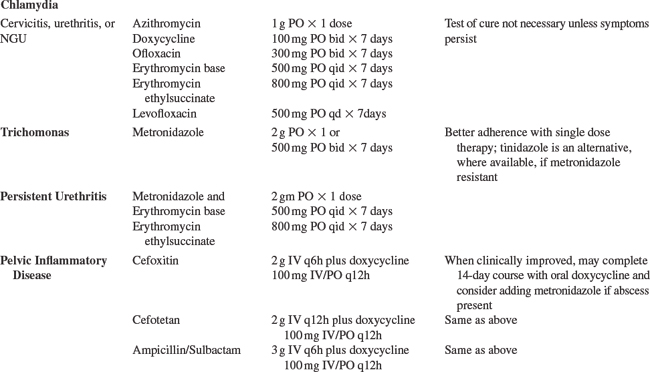
To diagnose LGV, rectal swabs can be tested for CT by PCR and if positive, the sample can be submitted for genotyping to identify LGV by the CDC’s chlamydia laboratory. A positive chlamydia serology is supportive of the diagnosis, and although not specific can help identify probable cases when genotyping is not available. The recommended treatment of LGV is doxycycline 100 mg for 21 days (Table 58-1).66
Genital Herpes–HSV-2
Epidemiology and Presentation of HSV-2 in the Setting of HIV-1
HSV-2 infection is one of the most common co-infections with HIV-1,176,192–194 occurring in 50–90% of HIV-infected persons, with the highest prevalence among African heterosexuals and MSM from the Americas.12,176,195–198 Anogenital herpes was one of the first opportunistic infections described in persons with AIDS.199 Among HIV-1-infected persons, especially those with low CD4+ T-lymphocyte cell counts, genital herpes can present with more severe, chronic herpetic lesions.85,200 Yet, as with immunocompetent individuals, most HIV-infected persons with HSV-2 are asymptomatic.201,202 HSV-2 increases HIV-1 acquisition10,18 and HIV-1 transmission.29,73,203 The effect of HSV-2 on HIV-1 transmission will be ascertained through an ongoing proof-of-concept ran-domized trial of HSV-2 suppression in HIV-discordant couples.
Diagnosis and Treatment of HSV-2 in HIV-Infected Persons
Clinical diagnosis of genital HSV-2 in HIV-1-infected persons is unreliable.77,80 An accurate diagnosis of HSV-2 can be made by culture or PCR of lesions or by IgG-based type-specific serology. Once a diagnosis is established, nucleoside analogs (acyclovir, valacyclovir, and famciclovir) decrease the frequency and severity of both HSV-2 recurrences and asymptomatic HSV-2 reactivation (Table 58-1). They are effective, safe, well-tolerated drugs in the setting of HIV-1 infection.204–207 Studies are underway to determine whether HSV-2 suppression decreases HIV-1 acquisition and transmission. Studies have already demonstrated that HSV-2 suppression significantly reduces HIV-1 levels in the blood which may slow HIV-1 disease progression, and in the genital tract which could result in decreased HIV-1 infectiousness,29,58,208–210 thus associated with both potential clinical and public health benefits (see Chapter 47 for more detail on HSV-2, HIV-1 co-infection).
NONULCERATIVE STIS
Nonulcerative STIs, specifically gonococcal, chlamydial, and trichomonal infections, have been reported to increase risk of HIV-1 acquisition, but to a lesser extent compared to the ulcerative STI.2,15 However, due to the high prevalence of these infections in some populations, nonulcerative STI may be a significant factor in the HIV epidemic globally. Nonulcerative STIs increase the risk of HIV acquisition in the range of 1.5- to 5-fold,1,2,11,14,15,211–214 perhaps due to recruitment of CD4+ T lymphocytes and increased levels of proinflammatory cytokines.
Nonulcerative STIs are also a risk factor for sexual transmission of HIV-1, after controlling for sexual exposure as demonstrated in African FSWs.14,215 In a nested case-control study of Zairian FSWs, women with gonorrhea (GC), CT, and Trichomonas vaginalis (Tv) had higher risk of HIV-1 seroconversion with an adjusted OR of 4.8 (95% CI, 2.4–9.8) for GC, 3.6 (95% CI, 1.4–9.1) for CT and 1.9 (95% CI, 0.9–4.1) for Tv.215 Nonulcerative STIs are associated with increased numbers of CD4+ T-lymphocyte inflammatory cells in the endocervix.216 Women with cervicitis have higher HIV-1 RNA concentrations in cervical secretions,50,54,56,217 and treatment for cervicitis results in significant decreases in cervicovaginal HIV-1 shedding.53,62 Similarly in men, nonulcerative STIs, particularly gonococcal urethritis, increase seminal HIV-1 levels61,71,218–222 and seminal HIV levels are reduced after effective treatment for urethritis.61,63
Neisseria gonorrhoeae, CT and PID
Clinical Presentation of Gonorrhea, Chlamydia, and PID in Persons with HIV-1
The presentation of GC and CT infection in HIV-1-infected individuals is the same as in HIV-1-uninfected persons; they cause urethritis and proctitis in men and urethritis, cervicitis, and PID in women. Less commonly, GC can also cause pharyngitis, conjunctivitis, or disseminated infection. PID, typically caused by GC, CT, or mixed aerobic and anaerobic organisms, can result in long-term sequelae in women, including infertility, ectopic pregnancies, and chronic abdominal pain. The microbiology and clinical presentation of PID do not seem to be altered significantly by HIV-1 infection.223 However, early studies reported a higher proportion of tubo-ovarian abscesses in HIV-1-infected women sometimes requiring surgical intervention, associated with a lower CD4+ T-lymphocyte count in one study.224–227 Of note, women with HIV-1 infection may not have an elevated white blood cell count at baseline as a diagnostic clue of PID.223,226 Given the possible increased likelihood of a tubo-ovarian abscess in HIV-1-infected women, clinicians should have a low threshold to do an abdominal ultrasound of an HIV-1-infected woman with lower abdominal pain and a presumptive diagnosis of PID.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree