Sex and Gender Across the Human Life Span: Introduction
- John Anderson, My Jo*
- John Anderson, my jo, John,
- When we were first aquent:
- Your locks were like the raven,
- Your bonie brow was brent.
- But now your brow is beld, John,
- Your locks are like the snaw;
- But blessings on your frosty pow,
- John Anderson, my jo.
- John Anderson, my jo,
- We clamb the hill thegither;
- And mony a cantie day, John,
- We’ve had wi’ ane anither;
- Now we maun totter down, John,
- And hand in hand we’ll go,
- And sleep thegither at the foot,
- John Anderson, my jo.
- Robert Burns, 1789
*Joy.
Do you recall this poem above from your high school English literature course? I certainly do, for its romantic depiction of a couple’s idealized journey together through this life and perhaps beyond. Little did I dream at the time that the central theme of this chapter would define a paradox that would provide a focus throughout my career in gerontology and geriatric medicine: women outlive men even as they experience greater levels of morbidity, health care utilization, and functional impairment throughout their lives.
A visit to almost any long-term care facility will prompt an obvious question from even the most casual observer: “Where are the men?” This chapter attempts to answer this intriguing question on the basis of both practical and theoretical considerations and extrapolate its conclusions and speculations to considerations of the health and social care of the elderly in this century of an unprecedented “age wave” in America and the world at large.
- What is the magnitude of the sex differential in longevity in the United States?
- How universal is this differential among different populations? Among ethnic groups in the United States? Among different nations, especially by degree of socioeconomic development?
- Has this differential always existed? If not, when did it emerge? And why?
- Are there genetic determinants of this differential? Are these mediated by sex hormones? How do these change across the life cycle?
What are the extragenital consequences of the sex differential in sex hormone physiology at various stages in the life cycle? What are these consequences, notably in the:
- Nervous system?
- Endocrine/metabolic system?
- Immune system?
- Cardiovascular system?
- Nervous system?
- What are the social, psychological, and behavioral implications of these differentials at various stages in the life cycle?
- What are the age-specific mortality rates of men and women (commonly expressed as the ratio between the two)—both all-cause and cause-specific?
- What are the age-specific morbidity rates in men and women—both all-cause (especially as expressed in functional status) and cause-specific?
- How do these differentials affect the lives of older persons, with specific implications for the duration and quality of life for elderly men and women? For the prevalence and duration of widowhood? For the health—physical, psychological, social, functional, and financial—and social and living circumstances of elderly widows and widowers?
- Are these trends changing? If so, how and why? And if so, what are the implications of those changes for the lives of elderly Americans in the twenty-first century?
- Finally, on a more macro level, what are the planning and policy implications of those changes (with special reference to health care and long term care)?
Definitions
Throughout this chapter the following definitions are used, conforming to the recommendations of the Committee on Understanding the Biology of Sex and Gender Differences formed by the Board on Health Sciences Policy of the Institute of Medicine of the National Academy of Sciences as explicitly advanced in their landmark 2001 report, edited by Wizeman and Pardue, entitled Exploring the Biological Contributions to Human Health: Does Sex Matter?:
- Sex: The classification of living things, generally as male or female according to their reproductive organs and functions assigned by chromosomal complement.
- Gender: A person’s self-representation as male or female, or how that person is responded to by social institutions based on the individual’s gender presentation. Gender is rooted in biology and shaped by environment and experience.
It is noteworthy that simply to clarify use of the terms sex and gender was one of the principal recommendations of this report. As will become evident, this distinction is important in consideration of the central issues in this chapter wherein sex and gender interact dynamically across the lifespan. This is evident perhaps most dramatically during periods of exceptional rates of change, notably during adolescence and across the menopause, transitions that will be highlighted in the discussion that follows. For both sex and gender contribute significantly to the longevity, function, and quality of life of elderly Americans and both must be considered in responsible planning efforts for their health and social care in the twenty-first century.
History of the Sex Differential in Longevity
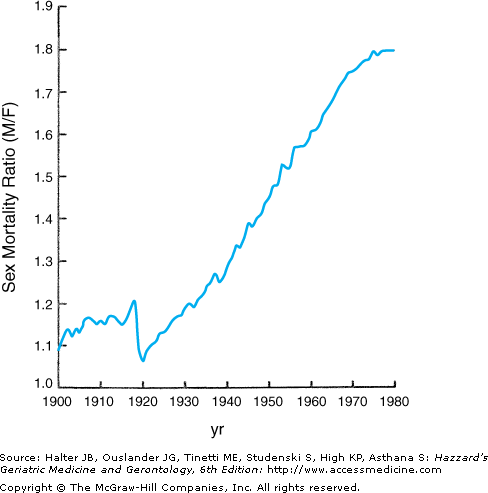
The sex differential in longevity from birth in contemporary American society is currently over 5 years (Table 45-1). However, this differential has changed dramatically over the past century and continues to evolve. At the beginning of the twentieth century, when the United States was still a developing nation, the sex differential in human longevity was just 2 years, and the overall age-adjusted male:female mortality ratio was only 1:1 (Figure 45-1). Throughout the first 70 years of that century, the ratio climbed steadily (except for a brief decline to near unity during the period 1918 to 1919, which was attributable to the [still unexplained] greater female mortality during the influenza pandemic of that era). This continuous increase translated into a sex differential in expected longevity at birth that reached a maximum at 7.5 years during the period 1969 to 1971. During those seven decades, the nation experienced progressive and dramatic socioeconomic development, a series of cataclysmic wars, and major changes in the roles of men and women in society. The social progress of women in the post-World War II and Vietnam war era and the rise of the feminist movement led some cynics (mostly men) to predict that women would begin to suffer the health and mortality consequences of the lifestyles and social roles historically considered as “masculine” (the so-called “they’ll get theirs” scenario). In this author’s experience, all persons, both lay and professional, have strong opinions as to why women outlive men. These opinions, however, are more often rooted in personal biases than objective scientific consideration. To illustrate this point, a 1996 survey of more than 500 college-aged students confirmed a distinct sex difference in attribution of the sex differential in longevity: young males ascribed the difference to the traditionally greater physical labor of men and the less stressful life of women, while the females attributed it to better self-care and attention to health by women.
LIFE EXPECTANCY (YEARS) | ||||
|---|---|---|---|---|
AGE | Males | Females | DIFFERENCE (FEMALE MINUS MALE) | PERCENT PERCENT DIFFERENCE* |
At birth | 73.8 | 79.5 | 5.7 | 7 |
At 65 yrs | 16.0 | 19.2 | 3.2 | 17 |
At 75 yrs | 10.0 | 12.2 | 2.2 | 18 |
While there is very little evidence to support any of the predicted deterioration in health or longevity of women in the present climate of changing lifestyles and occupational status (women in the workforce generally experience considerably fewer health problems, seek medical attention substantially less often, and carry less mortality risks than women who are not employed), there can no longer be any doubt that the sex differential in longevity in America has begun to narrow and, in retrospect, has been doing so progressively over the past 30 years. Harbingers of this trend were apparent in mortality data from the late 1960s, when a plateau in the previous linear rise in the sex differential in age-specific death rates began to emerge (see Figure 45-1), and the absolute difference between the sexes in expected longevity subsequently began to decline (see Table 45-1). However, as this trend has continued, it has reflected a greater gain among males than females (compare Table 45-2 with Table 45-1), even as both sexes have experienced continuing moderate increases to unprecedented average longevity. Should this pattern continue, it will introduce an important change in demography of the aging population, as the sex differential progressively narrows. This will reduce the burden of widowhood on survivors and hence the vulnerability of those survivors, especially widows, to loss of independence and consequent institutionalization (discussed further below).
LIFE EXPECTANCY (YEARS) | |||
|---|---|---|---|
Age | Male | Female | MALE/FEMALE RATIO, % |
At birth | 70.7 | 78.1 | 90.5 |
At 60 yrs | 17.5 | 22.4 | 78.1 |
At 65 yrs | 14.2 | 18.5 | 76.8 |
At 70 yrs | 11.3 | 14.8 | 76.4 |
At 75 yrs | 8.8 | 11.5 | 76.5 |
At 80 yrs | 6.7 | 8.6 | 77.0 |
At 85 yrs | 5.0 | 6.3 | 79.4 |
Nevertheless, a certain minimum sex differential in longevity appears to be a fundamental, perhaps immutable, principle of human gerontology. In America, this appears to hold true across ethnic groups with a broad range of average longevities. Nor is this phenomenon confined to the United States: the gap that developed in the United States in the twentieth century simultaneously emerged in all nations that underwent similar socioeconomic development (albeit with considerable variance in the magnitude of those changes; see Ory and Warner), with accompanying increases in average longevity of both sexes. Indeed, as detailed in the insightful review by Austad, women outlive men in 103 of the 104 demographic units tracked in the United Nations Demographic Yearbook, the lone recent exception being Bangladesh. These longevity increases are attributed principally to improvements in nutrition, education, housing, transportation, sanitation, public health, and health care, all leading to reductions in the diseases and conditions that predispose to the vastly premature deaths of infants and children. These developments also reduced the deaths of women of reproductive age associated with childbearing (including reduced numbers of pregnancies and deliveries). The same societies also generally experienced simultaneous advances in the status of women in general, a trend that appears to parallel reductions in premature mortality of women wherever it occurs (see Table 45-3 and concluding section).
Female specific: |
|
Male specific: |
|
When Does the Sex Differential in Mortality Begin?
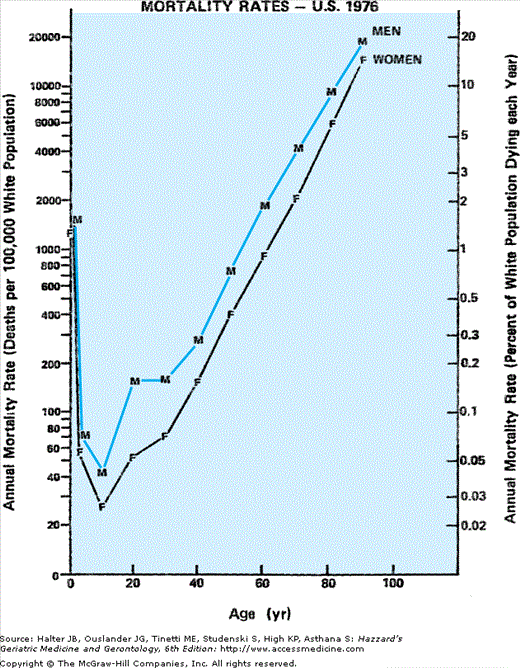
The sex differential in mortality (Figure 45-2) appears to begin at conception, when the ratio of male to female zygotes may be as high as 170 to 100 (for reasons that remain unclear, Y-bearing sperm are more likely to fertilize an egg than are sperm with the X-chromosome). Because of greater in utero male fetal death, however, by 10 to 12 weeks of gestation (as determined in abortuses), the gender ratio has declined to approximately 130:100. This decline in male survival continues throughout fetal life such that by birth this ratio has decreased yet further to 106:100 (see chapter by Neel in the volume edited by Ory and Warner).
This trend continues throughout the remainder of the human life span, albeit less dramatically with advancing age. And because the sex ratio of the population at every age reflects the cumulative survival of the sexes from each preceding birth cohort, the sex differential among survivors progressively favors females over males with every passing year of life. After the initial 6% excess of males over females at birth, the continued surplus of male deaths produces parity in numbers between the sexes during adolescence. However, at every age beyond that era, women outnumber men with an ever-growing sex disparity. This leads to a progressive rise in the ratio of women to men with advancing age, a pattern that appears to grow almost exponentially in old age, to a female:male ratio of approximately 3:2 among community-dwelling elderly persons older than age 85 years.
Somewhat paradoxically, this sex differential in the population grows with age in spite of a progressive narrowing in the gap in expected remaining longevity between the sexes, especially beyond middle age. This dwindles to little more than 2 years at age 75 years (see Table 45-2) and 1 year at age 85 years. Of practical utility to clinicians (e.g., in counseling aging couples), the ratio of remaining expected longevity between men and women remains relatively constant beyond middle age, stabilizing at approximately 80% (men compared with women) (see Table 45-1) even as the absolute difference narrows with each passing year of survival. Thus, a strategy designed to minimize the duration of widowhood should place special emphasis on survival of men through youth and middle age, the phases of life in which their greater vulnerability to premature death contributes most to the sex differential in longevity and protracted widowhood of their spouses.
By contrast with the situation among community-dwelling older persons, however, the sex ratio among residents of long-term care facilities (except, of course, those in the Veterans Affairs system) is far higher than 3:2. Ratios of 6:1 or 7:1 are not unusual in community nursing homes. This reflects in part the more vulnerable functional status of elderly women, as compared with men of similar age, and the common reality that they have outlived their husbands (even in nursing homes—in spite of the greater risk of death in the men in such facilities, measures of the performance of activities of daily living (ADLs) display lower average scores in female than male residents—one of many paradoxes raised in the sex/gender/longevity domain). Thus older women demonstrate greater musculoskeletal weakness and disability than do men of comparable age. They have more osteoporosis and are at greater risk to fractures from falls than their male counterparts. They also experience more urinary incontinence, an impairment that especially raises their risk of long-term institution utilization. Women older than age 85 years also have a greater prevalence of dementia, a pervasive disorder among the elderly that greatly increases their risk of institutionalization and often has devastating consequences to function and quality of life in the nursing home environment.
However, just as important, the social circumstances of elderly women, who often live alone, also contribute to their preponderance in long-term care institutions. As emphasized by Wylie, this reflects their more frequent single marital status: at the time of his review in 1980 approximately 80% of American men older than age 65 years were married, only 40% of women older than age 65 years were married, and the widow to widower ratio was 4:1, all figures that grew by each year of advancing age. Thus, given that for both sexes the person most likely to provide close and continuing support for a vulnerable older individual is the spouse, an elderly man who requires social and health care support to remain in the community is much more likely to have the help and companionship of his wife (who is also usually younger and more vigorous). However, an elderly woman who requires such support is much less likely to have an able spouse in attendance, often having outlived her husband, and she is a candidate for long-term institutional care unless alternative sources of social and health care support can be identified. Widowhood is the normative last phase of life for women: it often lasts more than a decade and is a reality that deserves serious consideration in responsible planning for the care and welfare of older persons for both individual couples and also as social policy.
This differential in caregiving needs and provision of care to elderly men and women is an example of how the terms sex and gender, as defined earlier, differ in a meaningful way: caregiving is culturally (as well as, arguably, genetically and hormonally) determined; the care provided by wives to their husbands represents an extension of their traditional and normative female nurturing role, a role that carries major implications for the care and welfare of aging persons, both male and female.
Mechanisms of the Biological Basis of the Sex Differential in Longevity
The universal nature of the sex differential in mortality across ethnic and geographic lines suggests that this phenomenon may be rooted in the biology as well as the sociology of the human species. The nature and possible biological mechanisms of this differential have recently been concisely summarized by Federman. As recently reviewed by Austad, examples from comparative zoology suggest that the greater longevity of the female versus the male generally predominates in the animal world. An important and instructive caveat prevails here, however: to observe this differential, the environment must be sufficiently protected as to mitigate the harsher consequences of “survival of the fittest” according to strict Darwinian principles, circumstances that prevail in the wild as well as during more primitive stages of human evolution. This cushioned environment, in turn, will permit survival of both sexes to an age approaching the maximum lifetime potential (MLP) of any given species. This is notably the case in zoos, in which aged animals are protected from inimical forces such as predation and malnutrition, and there females generally outlive males. As noted by Austad, in arguably the nonhuman primate most closely resembling the human, the chimpanzee, in zoos or similar protected environments females typically live longer than males by 3 to 4 years, a difference representing about 10% of their MLP. In this example, however, this reflects a dramatic decrease in male survival during relative youth (4–7 years of age), with parallel rates of survival thereafter.
Basic Molecular Genetics of Sex Differences
The following systematic review of potential mechanism that mediate the sex differential in human longevity (see Table 45-3) begins with the examination of the genetic platform of both sexes as concentrated in the sex chromosomes and upon which sex and gender dimorphism is played out across the life span and focus upon general biological regulators, notably levels of oxidative stress.
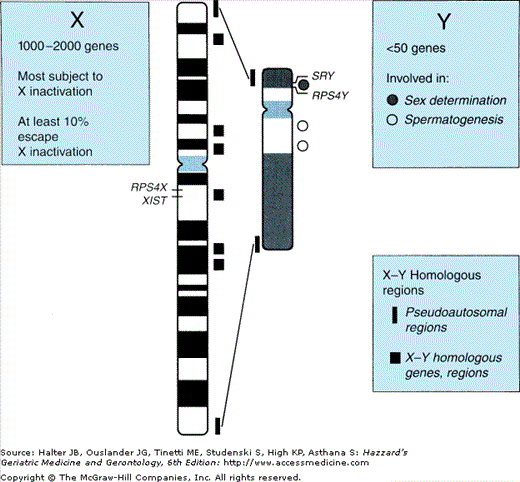
The sex chromosomes constitute but approximately 5% of the human genome (Figure 45-3). The Y chromosome is much smaller than the X chromosome and codes for only about two dozen different genes. Of those, the SRY gene determines development of the male gonadal phenotype, while spermatogenesis is related to a small number of other genes, mutation or deletion of which has been associated with certain cases of male infertility. Another class of Y chromosomal genes code for ribosomal proteins in Y-bearing cells (RPS4Y in Figure 45-3), while homologous genes on the X-chromosome code for those proteins in XX cells; thus certain ribosomal proteins throughout the organism differ between male and female cells. However, the functional consequences of these differences remain to be determined.
Male and female genomes differ in another important respect: females have twice the dose of X-chromosomal genes (i.e., “heterogametic”). This difference has led to the heterogametic sex hypothesis that the lack of a second X-chromosome in the male underlies at least a portion of his diminished longevity compared with the female. Here information from comparative zoology offers tantalizing mechanistic insight; for example, in the bird, the female has one long and one short sex chromosome and is thus the heterogametic sex (while the male has the two long [“Z”] sex chromosomes). According to the heterogametic hypothesis, male birds would be predicted to have a longevity advantage over females—which as cited by Austad is indeed the case for several species listed (budgerigars, zebra finches, and Japanese quail)—once again, however, explicitly specific to those raised in captivity.
In the human the X chromosome has approximately 160 000 DNA base pairs encoding an estimated 1000 to 2000 genes (see Figure 45-3). Only a few of these, the “pseudoautosomal,” have homologues on the Y chromosome. Products of these genes, like those on the autosomes, play a role in virtually every aspect of cellular function, metabolism, development, and control of growth and turnover. Especially germane to this discussion are those that play specific roles in particular tissues at particular points in development, several of which have been demonstrated to play critical roles in gonadal differentiation.
The twofold increase in X-chromosomal genes in females is in general offset by X-chromosomal inactivation, a complex, carefully regulated process unique to female cells marked in each by one of the two X-chromosomes becoming heterochromatic (identifiable on microscopy as the Barr chromatin body in the nucleus of female cells). This process renders genes on the inactivated X-chromosome functionally silent. It occurs in every somatic cell of XX females but not in XY males. Thus, not only must XX cells maintain the state of cell-specific X-inactivation throughout life but also, because the same X-chromosome is not inactivated in every cell, XX females are “epigenetic mosaics.” Not surprisingly, the process of X-inactivation is itself controlled by multiple factors, including genes. However, this may vary widely; in some families, sisters may show nearly identical patterns of X-inactivation, whereas in other studies of female identical twins, such patterns differ widely within twin pairs.
Directly germane to the focus of this discussion, female mosaicism underlies the dramatic sex difference in the severity and risk of death from diseases that are transmitted in a sex-linked recessive mode. For whereas a mutation in a gene on the X-chromosome will be expressed in every cell of an affected male, only half of the cells of the female will experience the consequences of that mutation, and her heterozygous state may carry no functional significance. However, given the rarity of X-linked recessive diseases with major physiologic consequences (e.g., classical hemophilia), the aggregate contribution of all the premature deaths of males from such sex-linked autosomal recessive diseases to the sex differential in longevity is miniscule.
Perhaps more relevant, however (at least theoretically), it appears that in cells grown in culture that at least 10% to 15% of X-linked genes appear to be expressed from the inactive chromosome and hence have “escaped” X-inactivation, contributing to a net increase in activated X-chromosomal gene products in females. This differential X-inactivation may have implications for vulnerabilities to certain diseases in affected individuals; for example, the suspected relationship between gastrin-releasing peptide receptor and smoking-related lung cancer, which is released by both active and inactive X chromosomes, with elevated levels hypothesized to lead to increased risk of lung cancer in women smokers. And, at the purely theoretical level, is X-inactivation maintained constantly throughout life? Could reactivation of the silenced X provide “back-up” genetic resiliency for women in later life?
Sex Differentials during Fetal, Childhood, and Adolescent Development
All humans—XX, XY, or with an atypical sex chromosome configuration—begin development from a common starting point in utero, with similar, phenotypically female genitalia until 6 to 7 weeks of gestational age. After that point expression of the SRY gene on the Y chromosome in males induces development of the testes. At about 9 weeks, this results in the secretion of testosterone. This, in turn, as modulated by multiple genes (at least 70 on sex chromosomes and autosomes), results in the development of the reproductive tract and masculinization of the male fetus as expressed most notably in the genitalia and the brain. In the absence of testosterone, the female phenotype is expressed; ovarian hormones are not required for this development. Moreover, animal studies and research on human dizygotic twins have suggested that testosterone secreted by a male fetus can exert a masculinizing influence on adjacent female fetuses, with anatomic, physiologic, and behavioral consequences. Thus events during intrauterine life, especially the secretion of testosterone by the fetal testis, exert powerful and enduring effects on postnatal life.
During late fetal life and infancy, however, the hypothalamic–pituitary–gonadal axis is largely suppressed, giving rise to relative hormonal stability throughout childhood (the “juvenile pause”). Adolescence, the gradual coming of age that transpires during most of the second decade of life, is the next phase of development during which the sex and gender differentials in growth and behavior are dramatic. Within that phase, puberty constitutes the transitional period between the juvenile state and adulthood during which the adolescent growth spurt occurs, secondary sexual traits appear (producing the dramatic sexual dimorphism of adult men and women), sexual activity is commonly initiated, and profound psychological changes occur. This tends to be conceptualized as a series of changes arising from reactivation of the hypothalamic–pituitary–gonadal axis.
However, despite the relative hormonal quiescence during the juvenile phase, small pulsatile patterns of follicle-stimulating hormone (FSH) and leuteinizing hormone (LH) can be detected as harbingers of coming adolescence in prepubertal children, and a striking eightfold difference in estradiol levels is demonstrable in girls. This is associated with a 20% advancement in bone age in girls versus boys and maybe related to their earlier onset of puberty. The development of breasts, which begins in white girls at an average age of 10.6 years, is under the control of estrogen, whereas growth in axillary and pubic hair is influenced by androgens secreted by the adrenals and ovaries. The age at which the benchmarks of pubertal development appear is also influenced by ethnicity (breast development begins about a year earlier on average in African-American girls than in white girls). Whether or not there has been an earlier age of breast development in recent decades remains a subject of controversy. However, contrary to much popular opinion, the average age of menarche appears to have remained constant for at least the past four decades. In boys, the beginning of puberty is marked by an increase in testicular size, which begins at a mean age of approximately 11 years (in both white and African-American boys).
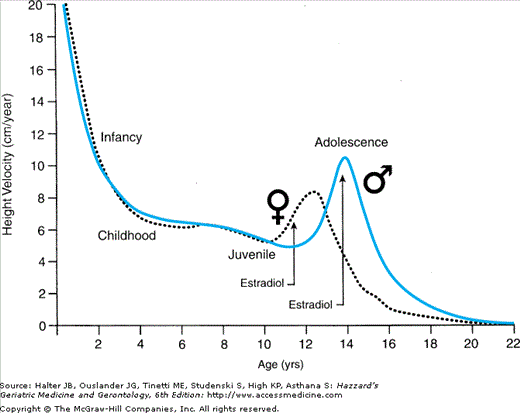
One of the most striking sex differentials associated with puberty is the earlier onset of the growth spurt in girls (Figure 45-4), in whom it may actually begin prior to breast development or growth of axillary or pubic hair. In boys, peak height velocity is reached approximately 2 years later than in girls, although boys are taller than girls at the onset of the spurt and finish, albeit later, with a mean height that is 12.5 cm (about 5 inches) greater. This differential reflects the greater prepubertal growth of boys and their taller status when their pubertal growth spurt takes off as well as their greater growth during the pubertal phase. The principal hormonal determinants of the growth spurt are growth hormone, insulin-like growth factor-1 (IGF-1) (hypothesis #3 in Table 45-3), and triiodothyronine in the prepubertal phase (accounting for 50% of the height increase during the subsequent pubertal phase), while estradiol is the main sex hormone involved in the pubertal phase in boys as well as in girls (the estrogen in boys arising principally by extragonadal conversion from testosterone).
Another hormonal feature of puberty is the phenomenon of adrenarche, which begins before age 8 years and is marked by progressive increases in adrenal androgen secretion and plasma dehydroepiandrosterone (DHEA) and DHEA-sulfate. Adrenarche is independent of the mechanisms that regulate the secretion of sex steroids from the gonads (gonadarche). Clinically adrenarche is signaled by the appearance of axillary and pubic hair and is considered premature when it occurs in white girls before age 7 years and in African-Americans before age 5 years. In contrast to the circumstance in boys, in whom premature adrenarche is a benign variant of normal puberty, in girls it is associated with a 10-fold increased risk of the polycystic ovary syndrome (PCOS) and its associated (and pathophysiologically important) features of ovarian hyperandrogenism and insulin resistance. These lead to an increased incidence of central obesity, type 2 diabetes, hypertension, and dyslipidemia (with a male-type pattern of higher low-density lipoprotein [LDL] and lower high-density lipoprotein [HDL] cholesterol levels prior to the era of the menopause), all cardinal features of the “metabolic syndrome.” PCOS, which has been estimated to occur in as many as 10% of women, appears to attenuate the sex-specific relative immunity to cardiovascular disease (CVD) otherwise enjoyed by women. As such, its understanding may provide special insights into the mechanism whereby men are at increased CVD risk, as compared with women, and the role of hormones, including insulin and sex hormones, in mediating that sex differential. Such insight may also provide opportunities to intervene with both behavioral and pharmacologic strategies to prevent premature CVD in men (and potentially in women as well, especially those with PCOS).
Of special relevance to the premise of this chapter that the sex differential in longevity originates in events in the life cycle long before old age, an intriguing body of evidence suggests that there is an association in girls between retarded in utero growth, the risk of premature adrenarche, and their subsequent development of PCOS; certain recent studies suggest that girls with antenatal growth retardation and low birth weights have fewer primordial ovarian follicles and a smaller uterus and ovaries at puberty and subsequent hyporesponsiveness to FSH, which may mediate (or at least be a marker for) the pathophysiology of PCOS. Thus, PCOS may be a classic example of a disorder of adolescence and adulthood that is programmed during fetal life but which holds important implications for health and longevity in middle and late life—as well as providing major insight into the general mechanism whereby nonandrogenized women enjoy longer (and more CVD-free) lives than their male counterparts.
These hormonal and physical changes at puberty also hold important implications for the sex differences in behavior during adolescence that are the subject of such intense contemporary public interest and concern. Some of these differences appear to proceed from the direct effects of gonadal hormones on the brains of adolescent boys and girls. For example, in studies of early adolescents, increasing levels of testosterone were associated with increasing aggression and social dominance in boys, while changes in estrogen levels correlated with changes in behavior patterns in girls during the pubertal transition. In various studies, certain behaviors appear to relate to absolute levels of sex hormones, others to the ratio of testosterone to estrogen, and still others to fluctuations in hormone levels. Still other studies reported changes in sex hormones in response to behaviors, such as increases in testosterone following athletic successes.
Such changes may have important, even life-altering, effects on the adolescents who experience them. Earlier maturation by girls, for instance, has been associated with social adjustment problems during puberty, earlier initiation of sexual activity and pregnancy, and later problems with eating disorders, depression, and substance abuse.
Boys may not have the same extent of social and behavioral problems, partly because of the recognition they receive from precocious physical strength and athleticism. However, there can be little doubt but that the risk-taking behavior of adolescent and young adult males contributes importantly to the stark contrast in mortality rates between young men and women (see below), and it seems logical to attribute such behaviors to hormonally mediated sex differentials across the adolescent transition. These become manifest not only in differentials mediated by sex in the sense of the definition urged by the Institute of Medicine report, but, importantly, also in how adolescents present themselves to the world and how its social institutions respond to them; that is, their gender manifestations. These issues are explored in depth in the provocative essay by Courtenay. Again the physical, psychological, behavioral, and social sex and gender differentials that present such stark contrasts during adolescence are rooted in the antecedent genetic, hormonal, and environmental lives of those boys and girls and also continue to modulate individual and group sex and gender differentials in health and longevity throughout adolescence and the remainder of the life span.
A practical consideration regarding the earlier maturation of girls than boys is also germane to the central theme of this chapter: that the duration of widowhood is an important determinant of the long-term care needs of elderly persons, especially women who have outlived their husbands. Because there is a normative sex differential in the age at marriage of bride and groom (generally about 2 years, likely reflecting the earlier maturation of females), this adds a commensurate period to the duration of widowhood, which may average nearly a decade (2–3 years difference in age at marriage plus approximately 5 to 6 years in the sex differential in longevity).
Sex May Affect Regulation of Redox State and Oxidative Stress
An attractive strategy in gerontological research examines mechanisms of homeostatic regulation that apply broadly across tissues and organ systems and which impact function across the life span. As such, they may converge with advancing age to become final common pathways that may mediate age-related comorbid diseases and ultimately frailty and demise in old age. One such mechanism that may contribute to the greater longevity of females is their reported superior regulation of redox balance and level of oxidative stress. As noted by Austad, this appears most evident in studies of rats (as apposed to mice), in which certain (notably the Wistar and Fischer 344) strains demonstrate substantially (ca. 16%) longer female longevity. Especially notable in this regard have been the reports from the laboratory of Vina, where studies of male (vs. female) rats demonstrated more oxidative DNA damage in isolated mitochondria. Yet more intriguing, these investigators reported that ovariectomy in females increased the production of oxygen-free radicals (hydrogen peroxide) in liver and brain mitochondria, an increase that was prevented by estrogen replacement. These findings were extended in measurement of endogenous mitochondrial antioxidant levels of glutathione peroxidase (mRNA and protein activity), which as predicted was higher in females than males. These authors interpreted these findings to attribute the greater longevity of female rats to attenuation of the age-related increase in reactive oxygen species (ROS) by estrogen, enhancing their antioxidant protection against age-related tissue damage. However, this provocative line of research clearly requires much further confirmation and investigation.
Sex Affects Neural Anatomy and Function
Another fundamental strategy of gerontologic investigation looks to biological integrating systems for insights into the aging process. This strategy examines how changes in those systems with aging render the organism progressively more vulnerable to insults from the environment in a stochastic cascade that limits survival of the individual and determines average and maximum longevity of a given species. Hence, consideration of the determinants of the sex differential in longevity (as well as of the causes of morbidity at various stages of the life cycle) have focused upon modulation of the neural, endocrine, and immune systems by sex hormones across the life span and how these might explain that differential.
As introduced in the immediately preceding sections and well-summarized in the 2003 Institute of Medicine report, fundamental differences in genetics and physiology between males and females result in important differences in neural anatomy and circuitry as well as in cognition and behavior at all points across the life span. While these differences begin during fetal life, they are amplified and modulated continuously at each age and each stage in a fashion that interacts dynamically with the environment, including sex and gender-sensitive interactions between children and their parents and other aspects of the different “male/masculine” and “female/feminine” cultures in which boys/men and girls/women develop and live.
These sex and gender differences can increasingly be demonstrated in the central nervous system and brain as investigative techniques in neuroanatomy, physiology, and neural imaging become more sophisticated. The brain is increasingly appreciated as an endocrine organ, too, with behavioral and anatomical consequences of exposure to hormones, including sex steroids, especially testosterone (e.g., greater aggression in male than in female mice). Such exposures are also being examined in relation to the gender identity of children. For example, case studies report that certain children with an XY-karyotype raised as girls because of mutilated or ambiguous genitalia may ultimately develop a male gender identity, presumably because of their intrauterine testosterone exposure. This challenges the belief that individuals are born with the potential to develop either male or female gender identity as determined by the sex of rearing.