47 Seminal Randomized Controlled Trials in Neuro-Oncology
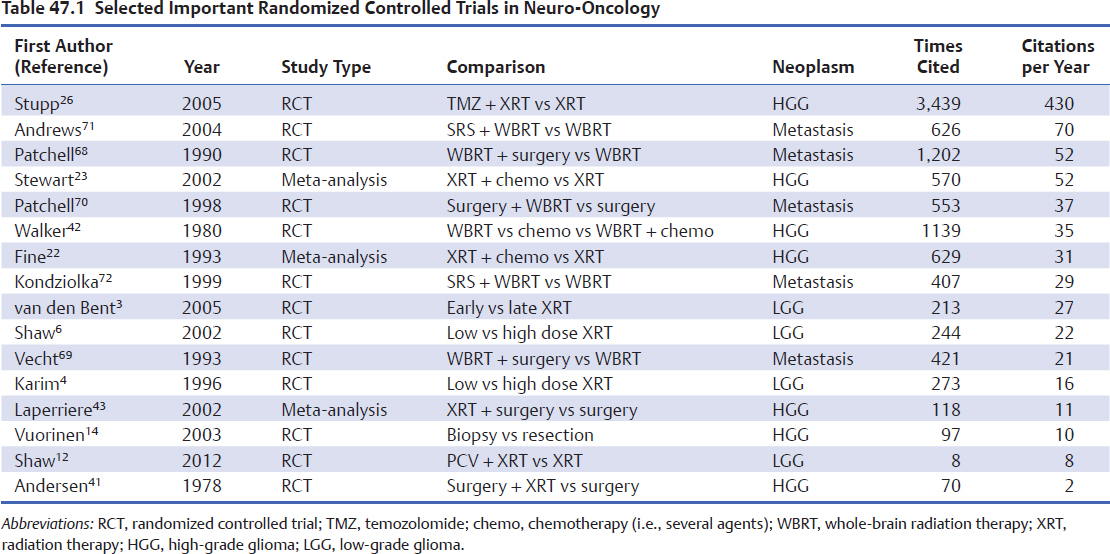
What is a seminal clinical trial? Important medical publications in any scientific field can prompt subsequent workers to build a superstructure of new knowledge on the foundations established by “seminal” studies that point out new directions for investigation. Perhaps a more characteristic function of important clinical trials is that they change clinical practice. These clinical studies move the field forward in individual and important ways. They have substantial and immediate academic impact: initial presentations in high-visibility venues such as international meetings, high citation rates by subsequent papers (Table 47.1), and important effects on the careers of those who designed and conducted the trials. They change clinical practice, as shown by population-based studies of changing patterns of practice and changes in treatment guidelines, and they change pharmaceutical practice by triggering regulatory approval for new agents or by finding new indications for drugs already approved for other uses. Finally, in many cases these trials include adjunct translational research that establish novel prognostic factors or biomarkers for response to the study drug.
Randomized controlled trials (RCTs) are the foundation of modern neuro-oncology. Their evidence guides important clinical questions in brain tumor surgery, radiation therapy, and chemotherapy. However, they compose a minority of the literature of neuro-oncology, which is replete with retrospective studies, nonrandomized prospective trials, and meta-analyses. Designing RCTs in neuro-oncology has historically been limited by the rarity of intracranial tumors at single institutions. In addition, as is the case for other solid tumors,1 relatively few adult brain tumor patients in the United States enter RCTs, perhaps 5% or less of patients with eligible brain tumor histologies. In contrast, pediatric neuro-oncologists enroll the majority of patients with eligible cancer diagnoses in clinical trials.2
This chapter briefly reviews several landmark trials in the treatment of patients with gliomas and metastases in view of their function as studies that advanced clinical knowledge and practice.
 Low-Grade Glioma
Low-Grade Glioma
Treatment options for histologically proven low-grade glioma (LGG) may include surgical resection, radiation therapy, and chemotherapy. Of these, radiation therapy and chemotherapy have been validated through an RCT to provide longer progression-free survival (PFS).
Radiation Therapy
The European Organization for Research on Treatment of Cancer (EORTC) trial 228453 randomized 314 patients from 24 centers to early postoperative radiation therapy (54 Gy in 1.8-Gy fractions) or to delayed radiotherapy at the time of progression. Although patients who received adjuvant radio-therapy had statistically significantly longer PFS (median PFS 5.3 years versus 3.4 years), overall survival was the same for the two groups (median 7.4 versus 7.2 years). Given the lack of an overall survival benefit, this RCT provides information that radiation therapy can be deferred until evidence of progression, especially given the potential toxicity of radiation leukoencephalopathy. EORTC trial 22844, an RCT that compared low-dose (45 Gy) and high-dose (59.4 Gy) radiation, showed no survival benefit with the higher dose, but a higher incidence of radiation necrosis in the high-dose treatment arm.4 In addition, the high-dose arm patients had lower levels of functioning on quality-of-life testing and higher symptom burden.5 These negative results were similar to those reported from a North American intergroup trial comparing 64.8 Gy to 50.4 Gy in 203 patients, with shorter survival and higher incidence of radiation necrosis in the high-dose group.6
Although EORTC trials 22844 and 22845 were disappointing from a therapeutic standpoint, showing no survival benefit for radiotherapy and higher symptom burden with higher doses, the data from these two trials were combined to establish an important system of prognostic factors for LGG. Five prognostic factors have been reported: age 40 years or older, astrocytoma histology, tumor diameter 6 cm or greater, the presence of neurologic deficits before surgery, and tumor crossing the midline. Each factor is associated with poor survival. A scoring system resulted in which two or fewer factors represent low risk and three or more represent high risk.7 The number of patients in the two protocols was sufficient to enable the prognostic system to be developed on the 22844 patient set and then validated on the 22845 patient set, a very robust design. The North American intergroup trial, which had confirmed the negative results of the EORTC dose-response trial, was then used to validate this prognostic scoring system.8
Pearl
• Randomized controlled trials show that radiation treatment at the time of diagnosis does not improve survival for adult patients with LGG and that treatment toxicity is related to radiation dose.
Pearl
• Randomized controlled trials provide high-quality prospective outcome data not related to the investigational treatment.
Extent of Resection
Despite what many surgeons feel is a central role of initial cytoreductive surgery in the management of LGG, no RCT has yet been performed to test the value of resection on outcomes in this disease. Many prospective and retrospective nonrandomized studies, mostly single-center, have suggested a beneficial effect of extent of resection.9 The EORTC 22844 trial,4 an RCT that primarily assessed dose response in LGG radiotherapy, found a trend toward improved overall survival and PFS for patients undergoing gross total resection (90–100%) as compared with subtotal resection (50–89%) or biopsy (< 50%). A North American intergroup LGG radiotherapy dose-response trial also found significantly longer overall survival in patients with extensive initial resection.6
However, surgical resection was not the randomized treatment in these trials and it therefore cannot be concluded from their results that there is a causal relationship between resection and better outcome. This is because not all patients who entered these trials were acceptable candidates for total or even extensive surgical resection. The assignment of patients to resection versus biopsy is highly dependent on factors such as tumor size and location, including involvement of eloquent cortex, both of which have been shown to be important factors in LGG prognosis.7,10 This bias in treatment assignment, known as confounding by indication, hinders a valid outcome between nonrandomized groups. To date, a lack of equipoise between biopsy and resection on the part of most brain tumor surgeons has prevented any RCT comparing the two modalities in LGG.
In 2008, a cohort of LGG patients entered into a nonrandomized prospective trial was exploited to circumvent this obstacle in a novel way.11 The authors reviewed the outcome in patients from an observation-only arm of a prospective Radiation Therapy Oncology Group (RTOG) clinical trial that required neurosurgeon-defined gross total resection for entry into the study. Postsurgical scans were prospectively archived at entry into the trial. Because surgeons’ assessment of extent of resection is notoriously overoptimistic, many of these patients actually had residual disease on retrospective review of the postsurgical scans. Patients with even small amounts of residual tumor (> 1 cm) as defined by enrollment magnetic resonance imaging (MRI) had poorer long-term PFS than patients with true gross total resections.
Pitfall
• There are no randomized studies to definitively show that gross total resection is a positive factor in outcome of adult patients with LGG.
Chemotherapy
The recently published RTOG trial 980212 randomized 251 LGG patients who had undergone subtotal resection or who were more than 40 years old to receive procarbazine, chloroethylcyclohexylnitrosourea (CCNU; lomustine), and vincristine (PCV) after radiation therapy versus radiation therapy alone. Progression-free survival was significantly improved in the cohort receiving PCV. Although the initial trial report indicated no overall survival benefit, at the time of writing there is early notification of a survival benefit from PCV in a follow-up analysis of the trial (www.cancer.gov/newscenternewsfromnci/2014/RTOG9802. Accessed May 2, 2014). An earlier negative trial on CCNU in LGG was probably underpowered, including only 54 patients.13 Although several nonrandomized studies have suggested activity and favorable toxicity profile for temozolomide (TMZ), no published RCTs to date have tested TMZ in LGG.
 Malignant Glioma
Malignant Glioma
Current treatment for high-grade (malignant) glioma (HGG) involves cytoreductive surgery followed by concurrent chemotherapy and radiation therapy. Similar to the landscape of LGG trials, RCTs in HGG have been primarily focused on assessing the role of chemotherapy and radiation therapy, but, with one exception, not the extent of surgical resection.
Extent of Resection
Determining the treatment effect of resection in HGG is hindered by the same barriers as in LGG: biased assignment of patients to the treatment based on resectability, and unwillingness of most surgeons to consider randomizing patients with resectable HGG to biopsy alone. The single RCT assessing the benefit of HGG resection was reported in 2003.14 This study of 30 patients demonstrated a statistically significant overall survival benefit for elderly patients (> 65 years old) who underwent resection rather than biopsy (median survival after craniotomy 171 days versus 85 days after biopsy). No other RCT has been performed to address this critical question. The wealth of nonrandomized studies9 is inherently limited by biased assignment to resection.
Several available study designs could potentially circumvent this difficulty, including randomization to resection versus observation for resectable residual disease after initial HGG resection (“second-look” surgery) or nonrandomized comparisons adjusting for a measure of resectability.15 Indirect evidence can also be obtained from RCTs of surgical adjuncts intended to improve extent of resection. When a RCT shows a benefit for an intervention thought to act purely as a surgical aid, such as intraoperative MRI or fluorescence-guided surgery, this supports the value of extensive resection in improving outcome.
Pitfall
• There does not exist good class I evidence to support the role of aggressive surgery in the treatment of patients with malignant glioma.
A RCT of 5-aminolevulinic acid (5-ALA) in improving resection by rendering residual HGG directly visible to the surgeon under blue light was reported in 2006.16 All patients entering the trial were considered eligible for complete resection by enrolling surgeons. Fluorescence-guided surgery was superior to standard resection on the primary outcome measure of PFS; 65% of 5-ALA patients had imaging-complete resections compared with 36% of controls. A reanalysis of the study found improved survival for patients with complete resections regardless of treatment arm (16.9 months median survival after complete resection versus 11.8 months after incomplete resection), supporting more extensive resection as the reason for better survival.17 Small trials of other surgical adjuncts, such as neuronavigation and intraoperative MRI-guided resection, have shown similar trends toward both more complete resections and longer PFS.18 By noting the parallel between extent of resection (as “response” to surgery) and chemotherapy response, and studies linking response to chemotherapy and chemotherapy survival benefit in other solid tumors using meta-analysis,19–21 it is possible that surgical adjunct RCTs may eventually provide stronger support for surgical resection in HGG than is currently available.
Chemotherapy
A number of RCTs have been designed to address the role of chemotherapy in anaplastic astrocytoma and glioblastoma multiforme, as reviewed by meta-analyses in 199322 and 2002.23 These studies predating TMZ both found a modest, but clinically and statistically significant, improvement in overall survival when a variety of cytotoxic chemotherapeutic agents were added to the treatment of patients with HGG. The two meta-analyses included largely overlapping groups of studies (16 in one and 12 in the other), and the direction and magnitude of the overall treatment effect were similar in the two analyses. However, the earlier meta-analysis, based only on published reports of trial results, had suggested a larger and earlier survival benefit in young patients and those with grade III histology, with glioblastoma patients having no survival advantage from chemotherapy until 12 months after diagnosis.22 In contrast, the later analysis, which was based on updated individual patient data provided by the original trial investigators, showed no variation in treatment effect with either age or histology.23
Controversy
• Meta-analysis can identify small treatment effects not visible in single RCTs. Subgroup analyses should be treated cautiously and may only be valid in individual patient data meta-analyses.
These meta-analyses illustrate several important principles. First, the original RCTs of chemotherapy in malignant glioma were too small to demonstrate a convincing clinical benefit given the modest activity of the agents tested; six of 12 trials enrolled fewer than 200 patients. Second, meta-analysis of RCTs was useful in clarifying the presence and magnitude of the small but real benefit of chemotherapy. Third, subgroup analyses in meta-analyses should only be attempted when individual patient outcome data from the original trials are available.24,25
In 2005, RCT of 573 patients with newly diagnosed glioblastoma who had undergone surgical resection demonstrated a survival benefit with concurrent TMZ administration when added to focal external beam radiotherapy (median survival 14.6 months versus 12.1 months for radiation therapy alone).26 A companion paper from the study demonstrated a larger benefit from TMZ in patients with methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter.27 Both conclusions were confirmed by the final report of the study in 2009.28 This study has been extensively cited by later papers (Table 47.1) and had a clear, immediate effect in changing clinical practice. Temozolomide was approved by the U.S. Food and Drug Administration (FDA) for adjuvant use in newly diagnosed glioblastoma 4 days after the study’s publication date (http://www.cancer.gov/cancertopics/druginfo/fda-temozolomide
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree