33 Skull Base Meningiomas and Other Tumors
A wide range of tumor pathologies occur at the skull base. They are often slow-growing, benign, extra-axial tumors that cause symptoms by involvement of the cranial nerves or a mass effect on the brainstem and cerebellum. They are frequently located in critical areas with brainstem compression and involvement of delicate neurovascular structures that are especially hard to reach with routine surgical techniques. The introduction of microsurgical techniques heralded an era of technical advances that ultimately translated into decreased patient morbidity and mortality.
The 1980s witnessed the popularization of skull base techniques. The combination of microsurgical and skull base surgery techniques has allowed access to areas of the brain once considered inaccessible. The addition of neuromonitoring, neuronavigation, improved optics, and surgeon experience has advanced the field to its current state. Most benign skull base lesions once considered inoperable are now curable with excellent rates of morbidity and mortality. The addition of stereotactic radiosurgery has further supplemented the field, providing tumor control in lesions not dissectible from essential structures and in poor surgical candidates.
 Skull Base Meningioma
Skull Base Meningioma
Meningiomas occur frequently, constituting 20% or more of all intracranial tumors. Their incidence in community-based series and clinical series varies from 1 to 6 cases per 100,000 persons per year and on average is estimated to be around 2.6 cases per 100,000 persons per year.1 However, in autopsy-based studies, meningiomas are present in 2.3% of people,2 suggesting that many people harbor undiagnosed asymptomatic meningiomas.
The modern widespread use of magnetic resonance imaging (MRI) has resulted in a large number of these meningiomas being discovered incidentally. The asymptomatic incidentally discovered meningioma is a management challenge. Tumor progression in conservatively managed meningiomas is reported to range from 22 to 90% in natural history studies,3–8 with higher rates reported in studies using volumetric rather than linear growth analysis. Significant factors associated with a higher likelihood of progression are age < 60, large tumor size (> 25 mm), lack of calcification, associated edema, and hyperintensity on T2-weighted MRI sequences.4,6,9 Close follow-up is necessary, particularly of skull base tumors. In a study of the natural history of petroclival meningiomas, growth was noted in 76% of cases, with 63% of the growing tumors causing a functional decline.10 Prudent surgeons should have an understanding of this natural history, allowing them to avoid the potential morbidities of surgery in the patient who is likely to receive little benefit from intervention, while not withholding a potential surgical cure from the patient who would otherwise be devastated by the disease.
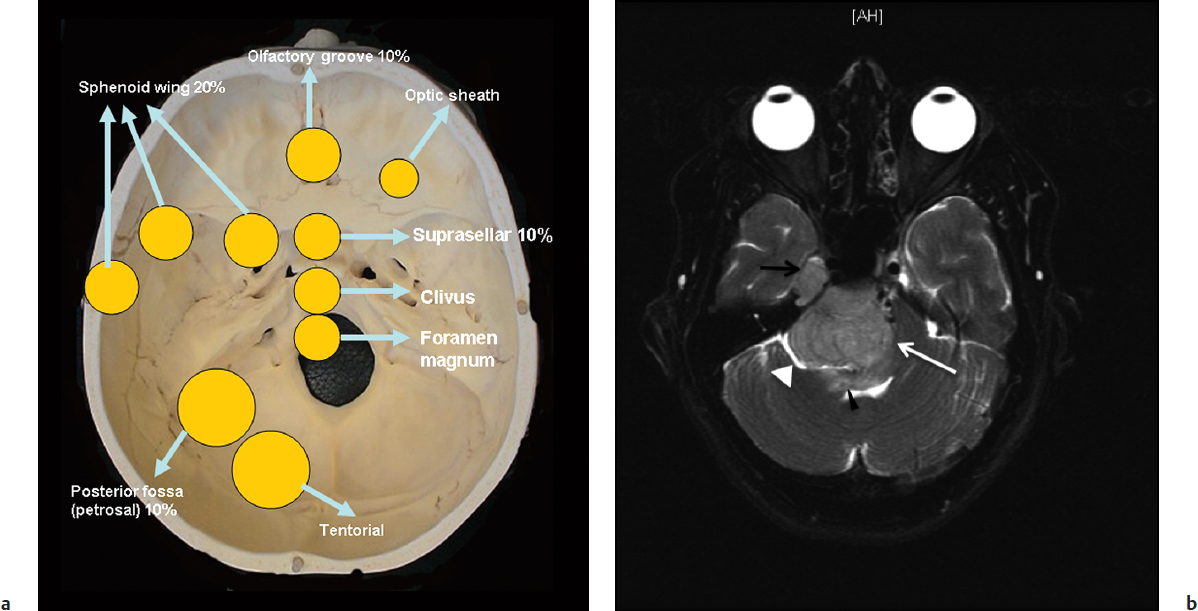
Forty percent of meningiomas occur at the skull base. The sphenoid ridge is the most commonly involved location at the skull base11 (Fig. 33.1a). Other locations include the olfactory groove, planum sphenoidale, tuberculum sella, anterior clinoid, cavernous sinus, cerebellopontine angle, clivus and petroclival area, and foramen magnum.
Imaging Studies
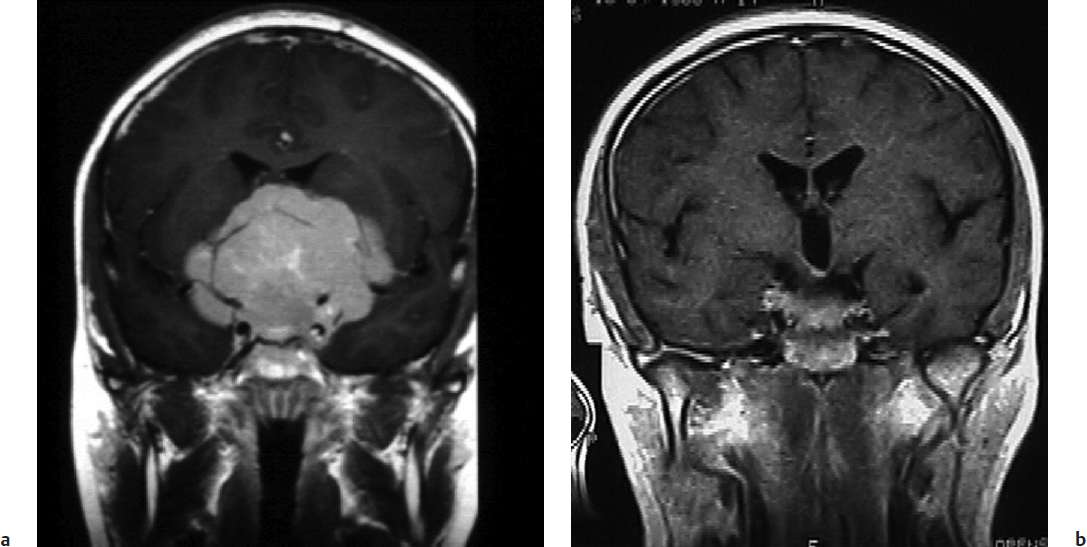
Computed tomography (CT) scan of meningiomas typically reveals a hyperdense extra-axial lesion relative to the adjacent brain. In up to 25% of cases, tumoral calcification may be present. In particular, bone sclerosis and hyperostosis may be seen at the site of origin of meningiomas of the skull base, which is believed to be tumor invasion. An MRI examination of these tumors typically demonstrates marked enhancement of the tumor with a dural tail. Mushrooming of the tumor is a concerning sign and has been associated with higher grade meningiomas and higher likelihood of recurrence.12,13 Imaging should be carefully reviewed for multiple lesions, particularly at the skull base. On T1-weighted images most of these tumors are isointense as compared with gray matter. On T2-weighted sequences, these lesions typically demonstrate similar or increased intensity relative to the gray matter. The T2 sequence is also useful in identifying a hyperintense band between the tumor and the brain. Absence of this band is a concerning sign and often represents an inability to dissect the tumor safely from the brain (Fig. 33.1b). Brainstem edema seen on T2 sequences is a troublesome sign and may be associated with poor outcome (Fig. 33.1b). MRI and magnetic resonance venography (MRV) can examine the extent of dural sinus involvement and sinus patency. Angiography may be indicated for preoperative embolization of feeding vessels supplying vascular tumors. Angiography also helps in the preoperative evaluation of the venous complex of the vein of Labbé, especially when the petrosal approach is indicated or when the tentorium needs to be cut. This venous anatomy can adequately be evaluated with MRV or computed tomography venography (CTV), but angiography may still be utilized in special cases.
Special Consideration
• The recurrence rate of meningiomas is directly related to the extent of resection.
Surgical Management
The mainstay of meningioma management regardless of location is surgical resection. The steps involved in resection of basal meningiomas include tumor devascularization via coagulation of the tumor base followed by internal debulking and finally removal of the tumor capsule from the surrounding vital neurovascular structures while respecting the arachnoid plane (Fig. 33.2). Clinical features that affect the extent of re-section include tumor location, size, consistency, and vascular/neural involvement. It is important to excise not only the neoplasm but also the involved dura, soft tissue, and bone to decrease the risk of recurrence. The extent of meningioma resection is directly associated with the risk of tumor recurrence. The extent of meningioma resection has been graded based on the Simpson classification. Grade I or II is radical tumor removal with resection of the involved dura and bone at the tumor origin (grade I) or coagulation of the tumor origin (grade II).14 The skull base approaches facilitate tumor re-section along with the involved bone and dura while placing minimal retraction on the brain and providing a flat wide surgical corridor to promote safe dissection of the vital neurovascular structures. A selection of these approaches along with the meningiomas for which they are well suited are described below.
Tuberculum Sellae, Olfactory Groove Meningiomas, and the Supraorbital Approach
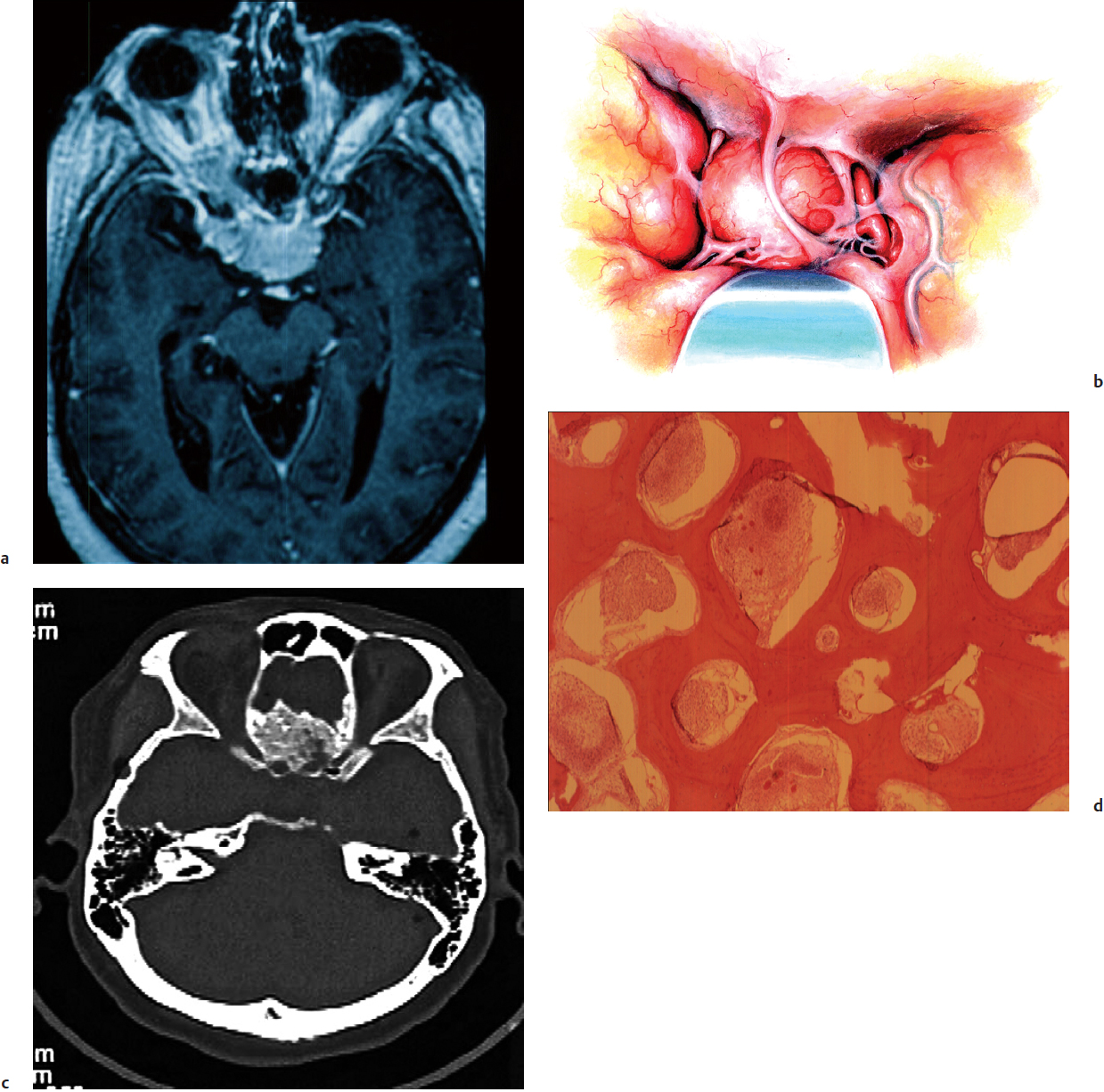
Tuberculum sella meningiomas comprise 5 to 10% of intracranial meningiomas (Fig. 33.3a). They typically present with progressive asymmetrical visual loss, incongruous visual field defects, and rarely panhypopituitarism. Visual field defects are identified in 84% of patients. Seizures are present in 10%, and pituitary dysfunction in 9%. These meningiomas originate from the tuberculum sella, chiasmatic sulcus, limbus sphenoidale, and diaphragma sella, and have a small dural attachment. The bone may be involved with exostosis at the anterior margin of the sella. The optic nerves are usually elevated and displaced laterally. Tumor invasion into the optic canals was present in 67% of tuberculum sellae meningiomas. Unroofing of the optic canal is typically necessary for safe tumor resection and decompression of the nerve. The carotid arteries are displaced laterally. The pituitary stalk and basilar artery are usually displaced posteriorly and separated by the membrane of Liliequist, which often provides an excellent dissection plane. Outcomes are generally good, as in most cases a Simpson grade I resection is achievable (86% in the senior author’s [OA] experience). Also visual outcomes are typically excellent, with visual improvement reported in 70 to 80% of patients, and these improvements are often dramatic.15 A small subset of anterior cranial fossa meningiomas arises directly from the diaphragma sella. These tumors typically grow retrochiasmatically, compress the hypothalamus, and are challenging.16
Patients with olfactory groove meningiomas may present with symptoms and signs of frontal lobe dysfunction, including changes in mental status, particularly mood and motivation. They may have anosmia. The Foster Kennedy syndrome of anosmia, optic atrophy in the ipsilateral eye associated with papilledema in the contralateral eye, is uncommon but associated with olfactory meningiomas. These meningiomas are typically discovered late and may become very large prior to correct diagnosis. The tumor arises in the midline of the anterior fossa over the cribriform plate of the ethmoid bone and the planum sphenoidale. The tumor’s blood supply is from the anterior ethmoidal artery. The tumors may extend into the ethmoids and nasal cavity.17
Controversy
• Expanded endonasal transsphenoidal approaches are applied in selected cases of anterior cranial fossa meningiomas.
The supraorbital approach (Fig. 33.3b), which includes the orbital rim with a frontal flap, is the authors’ preferred approach for tuberculum sellae and olfactory groove meningiomas. It provides a basal approach, eliminating the need for brain retraction, and affords the advantage of unroofing the optic canals bilaterally. The endonasal, endoscopic, and extended endonasal transsphenoidal approaches have been reported to remove smaller tuberculum sellae meningiomas (< 3.5 cm).18–28 To use this approach effectively, these tumors should have no vascular or neural encasement and present no extension into the optic canal.18,23 Several studies discussing the endonasal endoscopic approaches have shown relatively higher recurrence rates, in the range of 33%, as well as a greater proportion of patients with residual tumor (15 to 57%).20,21,25,27 Visual improvements ranged from 0 to 60% in patients with tuberculum sellae meningiomas treated via this approach.23,28 Worsening of vision was seen in up to 33% of patients.21 Series that limit the endonasal approach to carefully selected small midline lesions without major vessel encasement have demonstrated good results.21,26
The patient is placed in the supine position and the trunk is elevated 20 degrees, with the head moderately hyperextended and fixed in a Mayfield headrest to allow the frontal lobes to fall backward. The head is kept straight to facilitate orientation. The scalp incision is started 1 cm anterior to the tragus and continued behind the hairline to the level of the superior temporal line on the opposite side. In this manner, the superficial temporal artery course is posterior to the incision, whereas the branches of the facial nerve are anterior. The scalp behind the incision is elevated and freed from the pericranium, leaving the thick areolar tissue with the pericranium. A large pericranial flap based on the supraorbital and frontal vessels is then incised as far posteriorly as possible, dissected forward, and reflected over the scalp flap. Both layers of the temporalis fascia are incised posterior to and along the course of the upper branches of the facial nerve until muscle fibers are seen. The deep fascia, the fat pad, and the superficial fascia are then retracted anteriorly. The upper portion of the temporal muscle is detached from its insertion anteriorly and is retracted posteriorly, exposing the junction of the zygomatic, sphenoidal, and frontal bones.
The bone flap used depends on the size and location of the tumor. After the bone flap is removed, the dura is tacked up and opened under the microscope. Elevation of the frontal lobe should be minimal. The olfactory nerve is located and preserved by dissecting it for some distance from the base of the frontal lobe. Tumor feeders are encountered early; they are coagulated and severed on the basal aspect of the tumor. Devascularization is restricted to midline to avoid injury to the optic nerve on either side. Midline orientation is maintained by observing the position of the falx. The tumor is debulked with suction, ultrasonic aspirator, or a bipolar coagulator and microscissors.
Once the dissection approaches the neurovascular structures, only bipolar cautery and microdissection should be used. After the tumor is debulked, the optic nerves, which are displaced laterally, are identified. The tumor is slowly stripped from the flattened or engulfed nerve. Despite apparent encasement of or severe adherence to the nerve, a plane of dissection can be obtained under high magnification. To preserve any remaining vision, dissection of the optic nerve and its blood supply must be meticulous. Dissection may need to begin at the chiasm so that the surgeon can locate and dissect an obscured optic nerve on the opposite side.
Arterial structures should be preserved through the same method of sharp microdissection into an arachnoidal plane. The carotid artery is dissected free from the tumor with an array of microinstruments, including bipolar forceps, microdissectors, and scissors. Carotid dissection continues to free the ophthalmic artery, the posterior communicating artery, the anterior thalamic perforators, and the choroidal artery. Further dissection of the tumor progresses to the bifurcation of the internal carotid artery (ICA) and into the sylvian fissure. Dissection is then continued to free the middle and anterior cerebral arteries. In most cases, the tumor has simply displaced each vessel and their perforators, and rarely actually engulfs them. The A1 segments in particular are usually severely stretched or adherent and tend to tear. Although arterial twigs of the anterior cerebral arteries may supply the tumor, the surgeon must first be certain that these vessels are, indeed, tumor feeders and not hypothalamic perforators or the optic tract blood supply. Thus, each arterial branch should be dissected and followed to ascertain its eventual course. Particular precision is needed to spare the artery of Heubner and the vital branches to the striatum. As dissection continues, both A1 arteries and the anterior communicating artery are freed from the tumor.
The membrane of Liliequist is intact, making tumor removal from the posteriorly displaced basilar artery easy. The pituitary stalk can be recognized by its distinctive color and vascular network. A tumor extending backward under the hypothalamus usually displaces the pituitary stalk backward and to one side. Some tumors actually engulf the pituitary stalk and require meticulous and tedious dissection. The blood supply to the pituitary gland should be preserved. The tumor impinging on the hypothalamus can be removed gently if the surgeon maintains a plane of cleavage. Excessive downward retraction of the tumor, however, should be avoided. The arachnoid membrane of Liliequist provides an excellent plane of dissection for tumor removal. Often this membrane comes away with the tumor, leaving the rostral pons, midbrain, oculomotor nerves, and basilar artery and its branches in full view.
When the tumor extends into the optic canal, the anterior clinoid process, the roof of the optic canal, and the roof of the superior orbital fissure are drilled away with the diamond bit of the high-speed air drill. The dura is then opened along the optic nerve. Tumor tissue around the optic nerve is removed with bipolar forceps and microdissectors, and the surgeon must pay particular attention to preserving the hypothalamic and central retinal arteries. This bony drilling exposes the superior aspect of the cavernous sinus, and the ICA emerges through the superior wall and is surrounded and firmly anchored to the dura by a ring. Beginning at this emergence, an incision is made in the exposed dura and extended posteriorly toward the posterior clinoid process. The ICA is then followed in retrograde fashion into the cavernous sinus where it is dissected.
Following the removal of the tumor, its dural attachment should be resected or coagulated. Involved bone (Fig. 33.3c,d) should be removed with a diamond bit of a high-speed drill. Any opening into a paranasal sinus requires thorough repair of the dural defect. If the sphenoid sinus was entered, its mucosa is exenterated, and the sinus is packed with fat taken from the patient’s thigh. A large piece of fascia lata is laid intradurally and secured with sutures along the lesser sphenoid wing. The graft is then spread to cover the frontal fossa and then sutured to the frontal dura. The preserved pericranial flap in the frontal region is turned over the frontal sinus and extended over any defect in the floor of the frontal fossa. Titanium microplates are used here to reattach the bone flap to the cranial vault. The temporal muscle is sutured to the fascia at the lateral orbital rim, and the skin is closed in two layers.
 Clinoidal, Cavernous Sinus, and Meckel’s Cave Meningiomas, and the Cranio-Orbital Zygomatic Approach
Clinoidal, Cavernous Sinus, and Meckel’s Cave Meningiomas, and the Cranio-Orbital Zygomatic Approach
Clinoidal meningiomas originate from the anterior clinoid and present with visual loss in 84% of cases.29 They are divided into three groups based on their point of origin—thus the arachnoid relationship of the carotid artery and optic nerve with the tumor. Group I tumors originate proximal to the carotid cistern from the inferior aspect of the anterior clinoidal process. The carotid artery has a 1- to 2-mm course in the subdural space prior to entering the carotid cistern where it lacks an arachnoid membrane, and thus group I tumors adhere directly to the adventitia of the carotid artery, which makes it impossible to dissect the tumor from the carotid artery.30 Group II tumors originate from the superior or lateral aspect of the anterior clinoid process. This is distal to the arachnoidal investment of the carotid artery. The arachnoid investment of the carotid and sylvian cisterns enables these tumors to be dissected from the cerebral arteries. Additionally, for group I and II tumors, the optic chiasm and nerve are wrapped in the arachnoidal membrane of the chiasmatic cistern, and these tumors can be dissected safely from the optic apparatus.30
Group III tumors originate at the optic foramen and extend into the optic canal. They are typically small and cause early visual symptoms. The tumors occur proximal to the chiasmatic cistern, and therefore there may be no arachnoidal plane between the tumor and the optic nerve. The arachnoidal plane investing the carotid artery is usually present.30
Cavernous sinus meningiomas are usually of two general types: those that originate in and may be confined to the cavernous sinus, and those that invade the cavernous sinus but originate in an adjacent area. Meningiomas originating from the cavernous sinus and confined to it, present with extraocular movement disorders and facial paresthesias. Their management is controversial, with options including surgery, radiosurgery, or observation alone. Cavernous sinus meningiomas with a significant extracavernous component resulting in neural compression should be considered for microsurgical resection. Also, tumors compressing the optic apparatus should be considered for surgical resection to prevent radiation-induced optic neuropathy.31 A 4-mm margin should separate the tumor from the optic apparatus to avoid this complication.32 Patients with tumors larger than 3 cm are not ideal candidates for radiosurgery.33 Radiosurgery series with acceptable long-term follow-up report 5- and 10-year progression-free survival rates of 87 to 95% and 69 to 73%, respectively. New cranial neuropathies occurred in 10%.34,35
In the senior author’s (OA) experience, gross total resection was obtained in 44% of patients (71 of 163),36 with recurrence in 7% following gross total resection. Others have reported tumor-free survival following complete resection of 87% at 3 years and 62% at 5 years.37 Cranial nerve morbidity is an important consideration for cavernous sinus meningiomas. Improvement in cranial nerve function occurs in 14% and new cranial nerve neuropathy occurred in 18%; however, this is often transient.38
Meckel’s cave meningiomas originate within the cave itself and are rarely confined to it. Patients with these kinds of tumors usually present with a petrous apex syndrome consisting of facial numbness or pain and diplopia secondary to a sixth nerve palsy. In rare cases, these tumors due not extend beyond the cave and they can be removed through an extended middle fossa approach. More commonly these meningiomas grow and extend anteriorly into the middle fossa and the cavernous sinus, proceed into the upper clivus and petroclival area, and are approached through the cranio-orbital zygomatic approach.17
Controversy
• Asymptomatic cavernous sinus meningiomas may be managed with observation, radiosurgery, or surgical intervention.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree