Drug
Form
Calcium content
High cost
Aluminum hydroxide
liquid, capsule, tablet
No
No
(not recommended)
Calcium acetate
capsule, tablet
Yes
No
Calcium carbonate
liquid, capsule, tablet, gum
Yes
No
Magnesium carbonate/
tablet
Low
No
calcium citrate
Sevelamer-HCl
tablet
No
Yes
Sevelamer carbonate
tablet, powder
No
Yes
Lanthanum carbonate
tablet, powder
No
Yes
Several studies showed that all medications currently used as phosphate binders are effective in lowering serum phosphorus levels. All these medications should be administered with the food to bind phosphate in the gastrointestinal tract. Aluminum hydroxide (labeled use as a gastric antacid medication) is a potent, low cost, phosphate binder, but concern about skeletal, hematological, and neurological toxicity led to a preferential use of calcium salts (carbonate and acetate) in the 1990s [26]. The most severe cases of aluminum toxicity occurred in patients whose dialysate was contaminated with aluminum, and that aluminum-based binders only played a secondary role. Moreover, the quantity of aluminum-based phosphate binders that is safe is unknown, therefore, as numerous alternative phosphate binders have become available, the long-term use of aluminum-based phosphate binders should be avoided.
The use of large doses of calcium-containing phosphate binders subsequently led to concerns about calcium overload because of a potential for generating a positive calcium balance, then new calcium-free phosphate binders were introduced, as sevelamer-HCl, sevelamer carbonate, and lanthanum carbonate. Some papers, comparing the use of sevelamer with the use of calcium-based salts, have shown to attenuate progression of arterial calcification in patients with chronic kidney disease stages 3–5 [27] and stage 5D [28, 29]. However, other more recent randomized controlled trials have not reproduced these results and have found high and similar rates of progression of vascular calcification in patients receiving sevelamer-HCl as compared with those receiving calcium acetate [30, 31].
Recently some authors reported reduction in death for all causes and for cardiovascular disease in incident hemodialysis patients treated with sevelamer versus calcium carbonate [32]. Guidelines suggest restricting the dose of calcium-based phosphate binders in the presence of arterial calcification, adynamic bone disease or if serum PTH levels are persistently low.
In efforts to control hyperphosphatemia, dialysis regimens that allow an increase in phosphate removal may be an alternative in patients who cannot tolerate phosphate binders or are not willing to take sufficient amounts of them.
Nocturnal prolonged-duration hemodialysis, or daily (six times weekly), found significant decreases in serum phosphorus compared to standard hemodialysis given thrice weekly for 4 hours each session.
The renal population usually show low serum calcium levels as a consequence of vitamin D deficit. Guidelines [21] suggest that total serum calcium level, corrected for serum albumin level, should be maintained in the normal range (2.10–2.37 mmol/L) administering vitamin D sterols and calcium salt not close to mealtimes. In hemodialysis patients, serum calcium levels are corrected by calcium into dialysate. In the presence of persistent or recurrent hypercalcemia, it is strongly recommended that the dose of calcium-based phosphate binders and/or the dose of calcitriol or vitamin D analog be restricted.
KDIGO guidelines provide a suggestion to maintain PTH levels into the range of normality in chronic kidney disease stages 3–5 and between 2 and 9 times the normal range in chronic kidney disease stage 5D [21]. Active vitamin D receptor activators (VDRA) are one of the classic therapies suggested to achieve those PTH targets, by direct mechanism on VDRA expressed on parathyroid glands, and by indirect pathway, increasing serum calcium levels. Calcitriol (1,25-dihydroxyvitamin D3) is the natural VDRA historically adopted orally or intravenously each dialysis session to control PTH targets in renal patients. Classical side effects are hypercalcemia and hyperphosphatemia, due to increased intestinal absorption. Recently, four vitamin D analogs have been introduced in the nephrologic arena, namely, alfacalcidol, doxercalciferol, paricalcitol and oxacalcitriol.
Paricalcitol, derived from the side chain modification, elicits a selective activation of the VDRs expressed at the parathyroid gland with a lesser effect on those expressed in the intestinal tract [33]. Paricalcitol is administered orally or intravenously at the end of the dialysis session, and it has shown promising results in PTH value suppression in chronic kidney disease stage 2–5 [34]. Cinacalcet, the only clinically available calcimimetic agent, mimics an increase in levels of extracellular calcium, augmenting the signal caused by the binding of extracellular ionized calcium to CaSR (calcium sensor in parathyroid glands, cloned in 1993 [35]) to increase intracellular calcium and decrease PTH release; cinacalcet does not enhance intestinal calcium and phosphorus absorption.
Cinacalcet in secondary hyperparathyroidism treatment is approved only for dialysis population. Initial dosage is 30 mg/day orally, with titration up to the maximum dosage of 180 mg/day. As the main adverse event is hypocalcemia, its use is frequently associated with vitamin D analogs and calcium supplements. Weekly serum level check is requested. Nausea and vomiting are others of the most frequently reported adverse events in studies [36–38].
Emerging evidence of several pleiotropic effects related to the activation of the VDR is transforming the use of vitamin D sterols among nephrologists. Regardless of the PTH level, ergocalciferol, cholecalciferol, and calcifediol (nutritional vitamin D) are currently prescribed to replenish lower levels of circulating 25-(OH)-vitamin D when levels fall under 30 ng/mL in the community, as well as in chronic kidney disease and dialysis patients.
The early replenishment of nutritional vitamin D deficiency could be preventive, delaying the further onset and evolution of secondary hyperparathyroidism.
17.7 Hyperparathyroidism After Renal Transplantation
Renal transplantation is the optimal treatment in patients with CKD as it may correct most uremic metabolic and endocrine imbalances.
Notwithstanding the striking results obtained in recent decades in the improvement of both patient and graft survival and quality of life, some clinical problems in the post-transplant period are still waiting a better solution.
Among these, hyperparathyroidism remains one of the more disturbing because of its implications not only on the graft outcome, but also on the morbidity and mortality of the recipient.
As a consequence of a successful renal transplant most recipients recover the capability to produce 1,25-dihydroxyvitamin D3, normalize the phosphorus and calcium serum levels, and they are expected to deactivate the previous secondary hyperparathyroidism. This favorable outcome, however, does not occur in all recipients and a substantial percentage of grafted patients develop a so-called “persistent hyperparathyrodism”.
Patients with a long-standing history of secondary hyperparathyroidism, especially if inadequately treated during their dialytic phase and with the presence of a nodular hyperplasia/adenoma in the parathyroid glands, are at higher risk to develop persistent hyperparathyroidism.
In fact, the adenomatous cells have a low density of VDRs [39], CaSR [40] and FGF/klotho receptors [41], which are all factors that support the persistence of a pathologic autonomy of parathyroid glands. The post-transplant scenario may be further complicated when the achieved post-transplant GFR remains suboptimal, often <60 mL/min, a value that coincides with the trigger of bone and mineral metabolism disorders in CKD. A suboptimal synthesis of calcitriol and decreased intestinal calcium absorption induced by corticosteroids may play a role as additional factors of activation. For these reasons, PTH levels remain elevated in a percentage of recipients that varies between 20 and 50% according the different studies [42, 43].
This form defined as persistent hyperparathyroidism may switch to a tertiary hyperparathyrodism (term first coined by Goar, in The New England Journal of Medicine in 1963, to indicate the functional autonomy of parathyroid glands with adenoma in a chronic uremic patient) even many years after the renal transplantation. The metabolic and clinical consequences of the persistent parathyroid over-activity may have different aspects and targets.
Hypophosphatemia. Elevated PTH and FGF-23 levels, for their phosphaturic effect, are the main cause in combination with steroids and diminished intestinal absorption of severe hypophosphatemia, which is as a rule (up to 90%) as the transient form in the early post-transplant period. The main consequence of post-transplant persistent hypophosphatemia is a significant and progressive bone loss that increases the multifactorial risk of bone fractures in transplanted patients. Muscular weakness is also reported.
Hypercalcemia. It is the most important metabolic manifestation of persistent/ tertiary hyperparathyroidism even if other factors may play a role as resolution of soft tissue calcifications, immobilization, high doses of steroid treatment and hypophosphatemia. The more frequent clinical manifestations are: abdominal pains, renal stone formation, psychological disturbances including depression, cardiac arrhythmias, calciphylaxis and neurological symptoms.
Acute pancreatitis. It is a serious complication with a 3% incidence rate, characterized by a high mortality. Tertiary hyperparathyroidism is one of the factors favoring this life-threatening complication even if it is not clear if the pancreatic cell damage is due to the high levels of PTH or the concomitant hypercalcemia.
Delayed graft function. Nearly 20–35% of the deceased donor kidney grafts incur a delayed graft function generally due to an acute tubular necrosis. The factors traditionally implicated in the pathogenesis of this complication are the ischemia-reperfusion injury, the drug toxicity, the age of the donor and the length of cold and warm ischemia time. Some authors, recently, have drawn the attention on the putative role that a severe hyperparathyroidism could play as an additional factor [44]. PTH, through its effect on its specific membrane receptors, is able to increase the calcium flow through the cell membrane of different tissues and changes in intracellular calcium concentration and compartmentalization have been demonstrated to be closely related to cellular ischemic damage [45].
Post-transplant bone disease. In many patients the general benefit of the metabolic endocrine resetting induced by a well-functioning graft do not meet with a parallel improvement of pre-existing bone disease. Approximately 7 to 10% of renal transplant patients may experience a fracture, mainly of the cancellous bone but also of the vertebrae, with a higher frequency in female and diabetic recipients. Moreover, nearly 60% have a significant reduction of the bone mineral content [46].
The main alteration in bone remodeling after renal transplantation consists of a decrease in bone formation and prolonged mineralization in the face of persistent bone resorption. Generalized or focal osteomalacia and adynamic bone disease [47], and also predominant high-turnover bone disease [48], have been reported in different studies.
The pathogenesis of such a multiform pathologic picture is, obviously, multifactorial but the post-transplant GFR, the dose of corticoids given and the degree of hyperparathyroidism post-transplant might have the greatest impact on the bone.
How to manage a persistent/tertiary hyperparathyroidism when refractory to the medical treatment remains matter of debate.
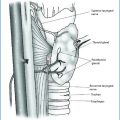
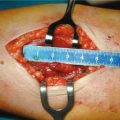
Parathyroidectomy is reported with a frequency ranging from 1.3 to 20% and is recognized as the most common indication of hypercalcemia and of PTH-mediated severe bone disease. Some authors [49] consider subtotal parathyroidectomy as the treatment of choice because, without including the economical aspects of long-life treatment with calcimimetic, it results in a permanent solution. The decision for a parathyroidectomy, however, must take into account the frequent deterioration of kidney function that occurs after the surgery. It remains an unsolved problem as the precise reasons are not clear.
Calcimimetics (cinacalcet). The introduction of calcimimetics has been a major breakthrough in the treatment of hyperparathyroidism in general and appears to be safe and effective also for the treatment in the post-transplant setting as well, where it is considered the alternative to surgical parathyroidectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree