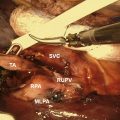
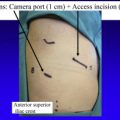
Lung cancer is a global health burden and is among the most common and deadliest of all malignancies worldwide. The goal of screening programs is to detect tumors in earlier, curable stages, consequently reducing disease-specific mortality. The issue of screening has great relevance to thoracic surgeons, who should play a leading role in the debate over screening and its consequences. The burden is on thoracic surgeons to work in a multidisciplinary setting to guide and treat these patients safely and responsibly, ensuring low morbidity and mortality of potential diagnostic or therapeutic interventions.
Key points
- •
The goal of screening programs is to detect tumors in earlier, curable stages, consequently reducing disease-specific mortality.
- •
The issue of screening has great relevance to thoracic surgeons, who should play a leading role in the debate over screening and its consequences.
- •
The burden is on thoracic surgeons to work in a multidisciplinary setting to guide and treat these patients safely and responsibly, with low morbidities and mortalities of potential diagnostic or therapeutic interventions.
Lung cancer is a global health burden and is among the most common and deadliest of all malignancies worldwide. In the United States, lung cancer accounts for more than 25% of all cancer deaths, exceeding deaths from breast, colon, and prostate cancers combined. More than 80% of individuals with lung cancer die of the disease, primarily because a large proportion of patients with lung cancer present with locally advanced or metastatic disease. Intuitively, early detection of resectable and potentially curable disease may reduce the overall death rate from lung cancer. Historically, screening for lung cancer was not recommended by most clinical societies and health care agencies in the United States. However, following the mortality benefit identified in the National Lung Screening Trial (NLST), published in 2011, most US guidelines now recommend screening with low-dose computed tomography (LDCT) in at-risk populations. This shift in policy can be expected to significantly increase the number of patients found to have lung cancer, but also those found to have benign lung nodules. Much of the current debate has now turned toward identifying the most appropriate “at-risk” populations for screening, ensuring appropriate management of screen detected nodules, and defining the optimum duration of computed tomography (CT) screening.
History of lung cancer screening
Chest Radiograph Screening
The first lung cancer mass screening project was conducted by Brett in London from 1960 to 1964. Although not a randomized trial, 55,034 men were assigned to undergo either chest radiograph (CXR) every 6 months for 3 years (the screened group), or a single CXR at the beginning of the study, followed by a repeat CXR at the end of the 3-year period (the “unscreened” group). At the end of the 3-year period, more lung cancers were detected in the screened group compared with the “unscreened” group (132 vs 96 cases). In addition, resectability was enhanced in the screened group. Despite these findings, lung cancer–specific mortality was not different between the 2 groups.
In the 1970s, the National Cancer Institute funded 3 randomized trials for lung cancer screening using both CXR and sputum cytology at Johns Hopkins, Memorial Sloan-Kettering Cancer Center, and the Mayo Clinic. Again, more cancers were found in the screened groups of patients, and resectability rates were significantly higher in the screened group. Nonetheless, again there was no statistically significant difference in the lung cancer–specific mortality between the screened and “unscreened” populations in any of the trials.
Early Computed Tomography Screening Studies
In the 1990s, increased resolution and data-acquisition speeds of modern CT scanners rekindled interest in screening for lung cancer. Initial findings from Henschke and colleagues of the Early Lung Cancer Action Project (ELCAP) showed that in a high-risk population, LDCT was superior to CXR in detection of lung nodules. Notably, 2.7% of those enrolled in the CT screening program had lung cancer, the great majority of which were stage I. A subsequent report by the International-ELCAP (I-ELCAP) group addressed overall curability estimated through 10-year survival rates of patients found to have stage I lung cancer by CT screening. The investigators reported an estimated 88% 10-year survival rate, markedly higher than survival rates predicted by the current staging system or among those presenting as a result of symptoms. They inferred that because CT screening leads to early detection of lung cancer and because those lung cancers found as a result of CT screening are curable, that CT screening leads to a reduction in lung cancer mortality. Several other groups subsequently evaluated CT screening for lung cancer. A review by Black and colleagues published in 2007 identified 12 studies, including 2 randomized and 10 single-arm observational studies. Significant variability existed in the study populations and in the definition of a positive finding in each. The percentage of positive screenings ranged from 5.1% to 51%. From baseline screenings, 1.8% to 18% of positive findings led to a diagnosis of cancer. Most of the tumors were stage I (53%–100%), with a high resectability rate (>78%). Only one of the studies reported 5-year survival: 76% for patients with cancer detected at baseline screening and 65% for patients with cancer detected at annual repeat scanning.
Screening for lung cancer with LDCT was not universally embraced, however. Bach and colleagues reported the findings from CT screening of 3246 high-risk patients from multiple institutions. The investigators reported a 3-fold increase in individuals diagnosed with lung cancer and a 10-fold increase in patients undergoing lung resection (compared with expected cases). They also found no evidence of a decline in the number of patients with advanced stages of disease or of deaths from lung cancer in the screened groups. The investigators concluded that CT screening may not meaningfully reduce the risk of dying from lung cancer and suggested that CT screening is inherently prone to overdiagnosis, thus exposing patients to unnecessary surgery. The study generated controversy given that the follow-up was relatively short (3.9 years) and that at least 1 of the 3 studies did not require the exclusion of symptomatic individuals, possibly undermining the core concept of screening.
Modeling Approaches to Estimate Mortality Benefit
Given the inability of early randomized trials and prospective studies to prove a mortality benefit to screening, others have attempted to address the magnitude of lung cancer mortality reduction using modeling approaches. McMahon and colleagues from the Mayo Clinic used 1520 current or former smokers undergoing CT screening to model predicted cases of lung cancer and deaths, which were compared with a simulated unscreened control arm. The model ultimately simulated 500,000 cases per study arm based on 5 annual screening examinations, to generate precise estimates of mortality. At 6 year follow-up, the screening arm had an estimated 37% relative increase in lung cancer detection compared with the simulated control arm and a 28% relative reduction in cumulative lung cancer–specific mortality. Although the model included many assumptions, such as lung cancer incidence rates, adherence to the screening protocol, and treatment by established guidelines, the study made a compelling argument for a mortality benefit from CT screening.
Similarly, Foy and colleagues used a lung cancer mortality model developed within the Cancer Intervention and Surveillance Modeling Network (CISNET) to address the potential for mortality reduction by CT screening for lung cancer. The comparison matched members of a CT screening trial (New York-ELCAP) with age, sex, and tobacco exposure–matched control patients from the CARET (Beta-Carotene and Retinol Efficacy Trial), with well-established lung cancer incidence rates. The simulation was repeated 5000 times to compare expected lung cancer mortality between the 2 groups. With the caveat of the inherent assumptions made for the purposes of modeling, the study suggested a 45.6% relative reduction in lung cancer mortality in the group of patients screened with CT.
More recently, a modeling study was performed by the CISNET on behalf of the US Preventive Services Task Force (USPTF). Models were created using deidentified data from the NLST and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial. The investigators judged a strategy of screening patients aged 55 to 80, with at least 30 pack-years, and no more than 15 years since quitting as the optimal scenario balancing benefits and harms. In this scenario, the estimated reduction in lung cancer morality is between 8.6% and 23.5%. All of these studies, albeit models, suggested that LDCT screening protocols, logically followed by earlier treatment of lung cancer, do likely provide a mortality benefit.
The Gold Standard: the National Lung Screening Trial
Between 2002 and 2009, the NLST enrolled 53,456 individuals between the ages of 55 and 74. All had a history of smoking of at least 30 pack-years and were either current smokers or former smokers who had quit within the past 15 years. Most individuals were male (59%) and younger than 65 years (73%). The trial compared low-dose CT screening (26,723) to CXR screening (27,733) using 3 annual screening rounds with 8 years of follow-up. In the CT screening arm, there were 354 deaths from lung cancer, compared with 442 in the CXR group, translating into a 20.3% reduction in lung cancer–related mortality. In addition, there was a 7% reduction in overall mortality in the CT arm of the trial. This absolute mortality reduction was unprecedented in the history of lung cancer screening and was greeted with much enthusiasm by advocates of CT screening. The NLST secondary analyses will continue to provide data over the coming years. In addition, several European randomized trials are comparing lung cancer CT screening with no screening. It is expected that pooling the results of these trials and the NLST will further clarify the role for CT screening and better define screening protocols. It should be noted that both the DANTE (Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays) and the DLCST (Danish Lung Cancer Screening Trial) trials failed to show a decrease in lung cancer mortality in the CT-screened patients; however, they were likely underpowered. The largest trial among these, the NELSON (Nederlands-Leuvens Longkanker Screenings Onderzoek) study, has not yet reported end results.
History of lung cancer screening
Chest Radiograph Screening
The first lung cancer mass screening project was conducted by Brett in London from 1960 to 1964. Although not a randomized trial, 55,034 men were assigned to undergo either chest radiograph (CXR) every 6 months for 3 years (the screened group), or a single CXR at the beginning of the study, followed by a repeat CXR at the end of the 3-year period (the “unscreened” group). At the end of the 3-year period, more lung cancers were detected in the screened group compared with the “unscreened” group (132 vs 96 cases). In addition, resectability was enhanced in the screened group. Despite these findings, lung cancer–specific mortality was not different between the 2 groups.
In the 1970s, the National Cancer Institute funded 3 randomized trials for lung cancer screening using both CXR and sputum cytology at Johns Hopkins, Memorial Sloan-Kettering Cancer Center, and the Mayo Clinic. Again, more cancers were found in the screened groups of patients, and resectability rates were significantly higher in the screened group. Nonetheless, again there was no statistically significant difference in the lung cancer–specific mortality between the screened and “unscreened” populations in any of the trials.
Early Computed Tomography Screening Studies
In the 1990s, increased resolution and data-acquisition speeds of modern CT scanners rekindled interest in screening for lung cancer. Initial findings from Henschke and colleagues of the Early Lung Cancer Action Project (ELCAP) showed that in a high-risk population, LDCT was superior to CXR in detection of lung nodules. Notably, 2.7% of those enrolled in the CT screening program had lung cancer, the great majority of which were stage I. A subsequent report by the International-ELCAP (I-ELCAP) group addressed overall curability estimated through 10-year survival rates of patients found to have stage I lung cancer by CT screening. The investigators reported an estimated 88% 10-year survival rate, markedly higher than survival rates predicted by the current staging system or among those presenting as a result of symptoms. They inferred that because CT screening leads to early detection of lung cancer and because those lung cancers found as a result of CT screening are curable, that CT screening leads to a reduction in lung cancer mortality. Several other groups subsequently evaluated CT screening for lung cancer. A review by Black and colleagues published in 2007 identified 12 studies, including 2 randomized and 10 single-arm observational studies. Significant variability existed in the study populations and in the definition of a positive finding in each. The percentage of positive screenings ranged from 5.1% to 51%. From baseline screenings, 1.8% to 18% of positive findings led to a diagnosis of cancer. Most of the tumors were stage I (53%–100%), with a high resectability rate (>78%). Only one of the studies reported 5-year survival: 76% for patients with cancer detected at baseline screening and 65% for patients with cancer detected at annual repeat scanning.
Screening for lung cancer with LDCT was not universally embraced, however. Bach and colleagues reported the findings from CT screening of 3246 high-risk patients from multiple institutions. The investigators reported a 3-fold increase in individuals diagnosed with lung cancer and a 10-fold increase in patients undergoing lung resection (compared with expected cases). They also found no evidence of a decline in the number of patients with advanced stages of disease or of deaths from lung cancer in the screened groups. The investigators concluded that CT screening may not meaningfully reduce the risk of dying from lung cancer and suggested that CT screening is inherently prone to overdiagnosis, thus exposing patients to unnecessary surgery. The study generated controversy given that the follow-up was relatively short (3.9 years) and that at least 1 of the 3 studies did not require the exclusion of symptomatic individuals, possibly undermining the core concept of screening.
Modeling Approaches to Estimate Mortality Benefit
Given the inability of early randomized trials and prospective studies to prove a mortality benefit to screening, others have attempted to address the magnitude of lung cancer mortality reduction using modeling approaches. McMahon and colleagues from the Mayo Clinic used 1520 current or former smokers undergoing CT screening to model predicted cases of lung cancer and deaths, which were compared with a simulated unscreened control arm. The model ultimately simulated 500,000 cases per study arm based on 5 annual screening examinations, to generate precise estimates of mortality. At 6 year follow-up, the screening arm had an estimated 37% relative increase in lung cancer detection compared with the simulated control arm and a 28% relative reduction in cumulative lung cancer–specific mortality. Although the model included many assumptions, such as lung cancer incidence rates, adherence to the screening protocol, and treatment by established guidelines, the study made a compelling argument for a mortality benefit from CT screening.
Similarly, Foy and colleagues used a lung cancer mortality model developed within the Cancer Intervention and Surveillance Modeling Network (CISNET) to address the potential for mortality reduction by CT screening for lung cancer. The comparison matched members of a CT screening trial (New York-ELCAP) with age, sex, and tobacco exposure–matched control patients from the CARET (Beta-Carotene and Retinol Efficacy Trial), with well-established lung cancer incidence rates. The simulation was repeated 5000 times to compare expected lung cancer mortality between the 2 groups. With the caveat of the inherent assumptions made for the purposes of modeling, the study suggested a 45.6% relative reduction in lung cancer mortality in the group of patients screened with CT.
More recently, a modeling study was performed by the CISNET on behalf of the US Preventive Services Task Force (USPTF). Models were created using deidentified data from the NLST and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial. The investigators judged a strategy of screening patients aged 55 to 80, with at least 30 pack-years, and no more than 15 years since quitting as the optimal scenario balancing benefits and harms. In this scenario, the estimated reduction in lung cancer morality is between 8.6% and 23.5%. All of these studies, albeit models, suggested that LDCT screening protocols, logically followed by earlier treatment of lung cancer, do likely provide a mortality benefit.
The Gold Standard: the National Lung Screening Trial
Between 2002 and 2009, the NLST enrolled 53,456 individuals between the ages of 55 and 74. All had a history of smoking of at least 30 pack-years and were either current smokers or former smokers who had quit within the past 15 years. Most individuals were male (59%) and younger than 65 years (73%). The trial compared low-dose CT screening (26,723) to CXR screening (27,733) using 3 annual screening rounds with 8 years of follow-up. In the CT screening arm, there were 354 deaths from lung cancer, compared with 442 in the CXR group, translating into a 20.3% reduction in lung cancer–related mortality. In addition, there was a 7% reduction in overall mortality in the CT arm of the trial. This absolute mortality reduction was unprecedented in the history of lung cancer screening and was greeted with much enthusiasm by advocates of CT screening. The NLST secondary analyses will continue to provide data over the coming years. In addition, several European randomized trials are comparing lung cancer CT screening with no screening. It is expected that pooling the results of these trials and the NLST will further clarify the role for CT screening and better define screening protocols. It should be noted that both the DANTE (Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays) and the DLCST (Danish Lung Cancer Screening Trial) trials failed to show a decrease in lung cancer mortality in the CT-screened patients; however, they were likely underpowered. The largest trial among these, the NELSON (Nederlands-Leuvens Longkanker Screenings Onderzoek) study, has not yet reported end results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree