Lobectomy is the gold standard treatment in operable patients with surgically resectable non–small cell lung cancer. Thoracoscopic lobectomy has emerged as an option for surgeons facile with the technique. Video-assisted thoracoscopic surgery (VATS) is used for a variety of indications, but its efficacy as a reliable oncologic procedure makes it appealing in the treatment of non–small cell lung cancer. Fewer postoperative complications and decreased postoperative pain associated with VATS procedures can lead to shorter lengths of stay and lower overall costs. Thoracoscopic surgery continues to evolve, and uniportal, robot-assisted, and awake thoracoscopic procedures have all shown promising results.
Key points
- •
Video-assisted thoracoscopic lobectomy has developed into a safe and effective treatment for lung cancer and is superior to lobectomy via thoracotomy in many regards.
- •
Development and further refinement of its technique has allowed thoracic surgeons to perform a wide variety of complex procedures in a minimally invasive fashion.
- •
With future improvement in optics, energy devices, and anesthesia management, the thoracoscopic technique will continue to serve as the pillar for development of thoracic surgical interventions.
Introduction
Pulmonary resection as treatment for lung cancer has been performed for more than 100 years. The first lobectomy for lung cancer was described in 1912, but pneumonectomy remained the gold standard for many years thereafter. In the 1960s, lobectomy became widely accepted as an oncologically sufficient operation. As technology advanced, thoracic surgeons took on the challenge of performing complete oncologic resections for lung cancer with minimally invasive video-assisted thoracoscopic surgery (VATS) in the early 1990s. The VATS approach to pulmonary resection has continued to evolve significantly over the last 25 years. In addition to pulmonary resection, thoracic surgeons are using thoracoscopy to perform complex operations on the airway, chest wall, mediastinum, esophagus, and even the certain cardiac procedures. Although there are no multicenter, prospective, randomized, controlled trials comparing pulmonary resection for lung cancer via thoracotomy and VATS, the minimally invasive approach has produced excellent outcomes and gained widespread acceptance.
Video-assisted Thoracoscopic Surgery Lobectomy as an Oncologic Procedure
Any operation intended to treat cancer should be undertaken with acceptable risk to the patient while also not compromising complete resection and staging. Since the introduction of the minimally invasive approach, many authors have evaluated VATS lobectomy for its oncologic efficacy, safety, reproducibility, and outcomes when compared with more conventional open techniques. In 1993, Walker and colleagues reported 158 cases of VATS lobectomy with 11% conversion rate, 1.8% mortality, and 3-year survival comparable to lobectomy with thoracotomy. As time went on and experience with the technique grew, more surgeons began to investigate all aspects VATS lung resection. Five years later, McKenna and colleagues reported the results of 298 patients who underwent VATS lobectomy, with a 6% conversion rate and 0.3% mortality. Port site tumor recurrence was reported in 1 case (0.3%). McKenna and colleagues followed this up with a review of 1100 patients who underwent VATS lobectomy and reported a 2.5% conversion rate, 0.8% mortality, 0.57% local recurrence, with a mean duration of hospital stay of 4.78 days. Onaitis and colleagues reported 500 VATS lobectomy cases with a 1.6% conversion rate, a 1% 30-day mortality, and no operative mortality. The 2-year survival for stages 1 and 2 non–small cell lung cancer was 85% and 77%, respectively. More recently, Berry and colleagues performed a retrospective review of nearly 1100 patients (610 VATS, 477 thoracotomy) undergoing pulmonary resection for lung cancer and showed similar 5-year survival between the 2 approaches. This was strengthened by a propensity-matched cohort of 560 patients within this group showing no significant difference between thoracotomy and VATS.
Proponents of the open approach to pulmonary resection for non–small cell lung cancer argue that the VATS approach limits the surgeon’s ability to adequately perform complete and accurate staging of the mediastinum, a key component of lung cancer staging and an important element of a sound oncologic operation. Mediastinal lymph node dissection during VATS lobectomy has shown to be equal to open lobectomy by D’Amico and colleagues. In this National Comprehensive Cancer Network’s database review, close to 400 patients undergoing VATS and open lobectomy were reviewed and there was no difference in the number of N2 stations and mean lymph nodes harvested. In 2 large metaanalyses, VATS lobectomy has been shown to be safe with a conversion rate of 1% to 2% and oncologic outcomes equal to open lobectomy.
Many surgeons who had initially trained solely in open pulmonary resection have taken these data and adapted their practices. Current thoracic surgery residents are gaining extensive experience with a wide variety of thoracoscopic procedures throughout their general and thoracic surgery training. Combined, these have led to increase in the number of lobectomies performed via a VATS approach across the world. In a recent review of the Society of Thoracic Surgeons General Thoracic Surgery Database, 45% of lobectomies were performed with VATS techniques.
Advantages of Video-assisted Thoracoscopic Surgery Lobectomy
In a propensity-matched analysis of a single-institution prospective database, Villamizar and colleagues evaluated 1079 patients undergoing VATS and open lobectomy over a 10-year period. Compared with open lobectomy, VATS lobectomy patients were shown to have fewer major complications, including atrial fibrillation, atelectasis, prolonged air leak, pneumonia, and renal failure. Duration of chest tube and length of hospitalization were shorter in the VATS lobectomy group. Similar findings were reported by Paul and colleagues in a review of more than 6000 patients undergoing lobectomy for non–small cell lung cancer from the Society of Thoracic Surgeons Database. In an even larger review of data from the Society of Thoracic Surgeons database, Boffa and colleagues confirmed that patients with stage I non–small cell lung cancer undergoing resection fared better. In this study, patients who underwent thoracotomy experienced significantly more pulmonary complications (21% vs 18%), atrial arrhythmias (13% vs 10%), and were more likely to undergo transfusion (6% vs 4%) than those who were treated with VATS resection, although the mortality was similar. Even when compared with a total muscle-sparing thoracotomy, 1 surgeon found that duration of stay was less for patient who underwent VATS procedures.
VATS lobectomy has also been shown to facilitate delivery of adjuvant treatment, an important component of treatment for patients with advanced stage non–small cell lung cancer. Peterson and colleagues reported a higher percentage of patients receiving 75% or more of their planned adjuvant regimen without delayed or reduced doses after undergoing VATS lobectomy compared with patients who had open lobectomy (61% vs 40%; P = .03).
The cost of VATS lobectomy has been reviewed in a study of close to 4000 lung resections and found to be less compared with open lobectomy ($20,316 vs $21,016; P = .027). This study also found the risk of adverse events was significantly lower in the VATS group (odds ratio, 1.22; P = .019). Recently, Farjah and colleagues even showed that 90-day costs are lower with VATS lobectomy when compared with open technique, explained by decrease in incidence of prolonged duration of stay (>14 days) and less health care use after discharge.
There is also growing evidence to suggest that patient’s immune function is better preserved after VATS compared with thoracotomy, as documented by the release of proinflammatory and antiinflammatory cytokines, immunomodulatory cytokines, circulating T cells (CD4), and natural killer cells, as well as lymphocyte function.
Video-assisted Thoracoscopic Surgery Lobectomy: Impact on Other Pulmonary Resections
As the technique has evolved and experience and comfort with VATS lobectomy has grown, thoracic surgeons have used it in various subsets of patients with lung cancer who in the past may not have been considered candidates for a curative resection or would have required a thoracotomy. In patients with poor pulmonary function, advanced age and small peripheral tumors who cannot tolerate a lobectomy or a thoracotomy, VATS sublobar resection (segmentectomy or wedge) can be an attractive option. The lower risk profile of thoracoscopy has encouraged surgeons to investigate its safety in patients who are at higher risk owing to preexisting conditions, including chronic obstructive pulmonary disease. Linden and colleagues performed VATS wedge resection in patients with a mean forced expiratory volume in 1 second of 26% and reported a 1% mortality rate. To decrease the risk of local recurrence after VATS wedge resection, Santos and coworkers reported the use of brachytherapy mesh placement over the stapled lung margin, which led to reduction of local recurrence from 18% to 2%. In patients with forced expiratory volume in 1 second of less than 60%, Ceppa and coauthors reported a much lower incidence of pulmonary complications ( P = .023) in patients undergoing VATS lobectomy versus lobectomy with thoracotomy.
Technical and oncologic principles of VATS lobectomy, namely individual ligation of the supplying artery, vein, and bronchus, lymph node dissection, and resection of lung parenchyma with surgical staplers have also been applied to performing minimally invasive anatomic segmentectomy. Schuchert and colleagues reported on 225 cases of anatomic segmentectomy performed by VATS or thoracotomy. Length of stay (5 vs 7 days; P <.001) and pulmonary complications (15.4% vs 29.8%; P = .012) were improved significantly in patients undergoing VATS segmentectomy. Similar outcomes have been reported by multiple other authors with acceptable survival and local recurrence rates.
Berry and colleagues reported on a hybrid technique of VATS lobectomy with en bloc chest wall resection without rib spreading or scapula retraction. In this series, the technique of VATS lobectomy was used to achieve lung resection, which was followed by a small counterincision to remove the involved ribs en bloc. They reported a shorter length of stay ( P = .03) in 12 patients with this hybrid approach compared with 93 patients who had a thoracotomy.
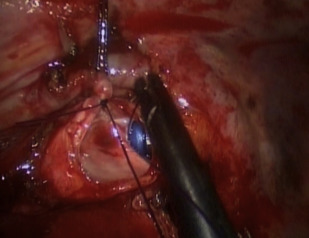
Further advanced VATS techniques such as bronchoplasty and sleeve resections have also been reported over the last 5 years ( Fig. 1 ). Agasthian reported a case series of 21 patients, 9 of whom had simple bronchoplasty; 8 patients had sleeve lobectomy and 4 patients had extended bronchial resection. All patients underwent hand-sewn closure of the bronchus with interrupted sutures. One patient developed bronchopleural fistula. There was no operative mortality and no local recurrence was reported at 6 months. Yu and colleagues reported on 9 cases from China undergoing VATS lobectomy and sleeve resection without any major intraoperative or postoperative complications.
Video-assisted Thoracoscopic Surgery Lobectomy: Development of Uniportal Video-assisted Thoracoscopic Surgery
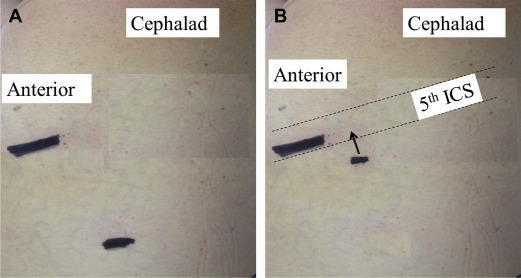
Over the years, VATS lobectomy has evolved and various modifications with 2 to 4 incisions have been reported by various leading surgeons across the world. Similar to single incision laparoscopic surgery, thoracic surgeons have evolved and VATS lobectomy has been modified into a single incision access with no rib spreading or multiple ports within the same intercostal space ( Fig. 2 ). Wedge resections to complex pulmonary resections have been reported. Over a 10-year period, Rocco and colleagues performed more than 600 uniportal VATS cases. The majority of these cases were for pleural disorders and wedge resections for pulmonary nodules. The authors reported excellent outcomes without any major intraoperative complications. Gonzalez-Rivas and associates reported their first 100 cases over a 2-year period with impressive results. The majority (96%) of lobectomies were accomplished with the uniportal technique, with no operative mortality. Mean chest tube duration and length of stay were 2 and 3 days, respectively. An average of 14.5 lymph nodes were harvested per resection with 154 minutes of mean operative time. Tam and colleagues reported similar results in 38 uniportal VATS lobectomy. Six patients required thoracotomy. In all, 97% of patients did not require intravenous analgesia and mean time to return to full normal activities was 7 days. Gonzalez-Rivas and colleagues have also reported uniportal right pneumonectomy without any major complications. McElnay and colleagues showed no difference in median morphine use or visual analog pain score in the first 24 hours postoperatively, or in patient-controlled analgesia duration, chest drain duration, or overall length of stay in 15 uniportal VATS lobectomies compared with the standard multiport technique.
Video-assisted Thoracoscopic Surgery Lobectomy: Development of Robotic Assisted Video-assisted Thoracoscopic Surgery
Advancement in robotic technology has generated interest among thoracic surgeons to its suitability for VATS pulmonary resections and other thoracic operations. It has been proposed that 3-dimensional optics and the articulation provided by robotic instruments may allow for increased use of a minimally invasive approach for pulmonary resection. The learning curve for robotic prostatectomy has been shown to be the same among laparoscopic trained fellows and experienced open surgeons who are not familiar with minimally invasive skills. This has lead thoracic surgeons to wonder if this experience can be replicated in thoracic surgery. Can surgeons not trained in VATS lobectomy perform robot-assisted VATS resection? More recently, the dual console systems, infrared technology for better anatomic visualization and tissue perfusion as well as improved simulation and training have made surgeons experienced in VATS lobectomy techniques interested in including robotics in their practice. Louie and colleagues compared directly robotic and thoracoscopic pulmonary resection in a case-control analysis of anatomic robotic and VATS lung resections: 46 robotic resections (40 lobectomies, 5 segmentectomies, 1 conversion to VATS included in this group for intention-to-treat analysis) were compared with 34 VATS resections (27 lobectomies, 7 segmentectomies). Length of stay, major and minor postoperative morbidity, and operative times were comparable. In a multiinstitutional retrospective cohort study of 325 patients who underwent robotic lobectomy, median chest tube duration and length of stay was 3 and 5 days, respectively. Major perioperative complications were seen in 3.7% of patients and surgical mortality was 0.3%. The estimated 5-year survival was 80%. Implementation of robotic surgery programs carry a high capital cost and require expensive maintenance protocols. In a recent study, Nasir and colleagues evaluated 862 robotic lung resections. The 30-day mortality was 0.25% and major morbidity was seen in 9.6% of patients. The authors estimated a profit of $4750 per patient after factoring in the operative and hospital cost. Median length of stay in this study was 2 days.
Video-assisted Thoracoscopic Surgery Lobectomy: Development of Awake Thoracoscopy
Traditionally, intubation with a double-lumen tube or use of an endobronchial blocker and single lung ventilation has been considered mandatory for thoracoscopic surgery to obtain optimal visualization. This is tolerated well in most cases, but adverse effects of general anesthesia and airway trauma from double-lumen tube placement are inevitable. Many thoracic surgery patients have preexisting comorbid conditions and cardiopulmonary compromise, which makes general anesthesia much more precarious. These issues have led some thoracic surgeons to explore the concepts of awake or nonintubated thoracoscopy. Pleuroscopy with drainage of effusion and pleural biopsy with local anesthesia has been performed routinely with flexible scopes in an outpatient setting for many years, mostly by pulmonologists. Anesthesia for a more complex thoracoscopic intervention, termed ‘awake VATS’ includes a regional block with or without conscious sedation. This consists of one of the following: local anesthesia, intercostal nerve blocks, paravertebral blocks, and thoracic epidural anesthesia. In this setup, open pneumothorax after trocar insertion leads to gradual collapse of the nondependent lung and leads to spontaneous 1-lung ventilation.
In a small, randomized trial performed by Pompeo and colleagues, 43 patients with spontaneous pneumothorax underwent VATS bullectomy and pleurodesis under a thoracic epidural anesthesia. Their results showed safety and efficacy of this technique of VATS along with shorter hospital stay and reduced cost. The same group has also reported 19 cases of empyema treated with awake VATS decortication. Three patients developed mild hypercapnia that resolved with time and 4 patients required general anesthesia because thick pleural peel required a nonemergent thoracotomy. Chen and colleagues reported their single institution experience of doing awake VATS in 285 cases. Of these, 137 were VATS lobectomy, 132 were VATS wedge resection, and 16 were VATS segmentectomy. Conversion to general anesthesia was required in 4.9% of cases and 1 patient required thoracotomy for bleeding. There was no operative mortality. Anesthesia consisted of thoracic epidural, sedation and temporary intrathoracic vagal blockade for inhibition of cough reflex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





