Chapter Outline
Plain Language Summary 242
Introduction 242
Background 243
Mammographic Breast Density 244
BD and Digital Mammography 245
Supplemental Screening Modalities for Breast Cancer Screening 247
Hand-Held Ultrasound of the Breast 247
Automated Whole Breast Ultrasound 248
Breast MRI 248
Digital Breast Tomosynthesis 248
Screening Hand-Held Breast Ultrasound (HHUS) Studies in Women With Dense Breasts 249
Japan Strategic Anticancer Randomized Trial 249
American College of Radiology Imaging Network 6666 Trial 249
Observational Studies 251
Summary: Screening HHUS of the Breast 251
Automated Whole Breast Ultrasound (ABUS) Studies in Women With Dense Breasts 253
Screening Breast MRI Studies 253
Summary: Screening MRI of the Breast 254
DBT Studies in Women With Dense Breasts 255
Summary 256
Who Should Receive Supplemental Screening? 257
List of Acronyms and Abbreviations 257
References
Plain Language Summary
Mammography is the only screening test that reduces death from breast cancer in randomized trials. However, it does not detect all cancers that are present. One of the reasons that mammography misses cancers is that dense areas on the image can obscure cancers. Both breast cancers and breast density show up as white on mammograms so it is more difficult to identify cancer in an area of density. Digital mammography improves the detection of breast cancers in dense breasts, but cancers are still missed. This raises the question about the potential benefits of additional screening with different technology.
Four approaches have been advocated to identify cancers in women with dense breasts and normal screening mammograms: hand-held ultrasound, automated breast ultrasound, breast magnetic resonance imaging (MRI), and digital breast tomosynthesis. All four of these technologies generate multiple images representing slices of the breast that allow the radiologist to visualize the breast with less overlapping tissue. This is particularly useful in dense breasts because overlapping dense tissue may hide breast cancer.
No clinical trials demonstrate that supplemental screening reduces death from breast cancer. Studies do show that all four techniques identify additional cancers in women with normal mammograms. Hand-held ultrasound is the best studied: it identifies an additional two cancers per 1000 women screened, but is time-consuming, dependent on the skill of the operator, and requires more biopsies to find one cancer than other techniques. Automated breast ultrasound was developed to remove the operator dependency, shorten the time of image acquisition, and improve the efficiency of ultrasound, but suffers from the same high number of biopsies performed to find one cancer. MRI finds the most additional cancers, but is very expensive, time-intensive, and requires intravenous contrast. It is only practical for women at the highest risk for breast cancer, such as carriers of mutations in the BRCA genes. Tomosynthesis, on the other hand, finds an additional two cancers per 1000 women screened and requires a similar number of biopsies to find one cancer as traditional digital screening mammography; however, as currently practiced it doubles the radiation dose. It is the most promising technology.
Not all women with dense breasts require additional imaging. Breast density information can be combined with a woman’s risk for breast cancer to target those women at highest risk for missed cancers. Using a risk-based strategy, only about one in four women with dense breasts would be recommended for supplemental screening.
Introduction
This chapter summarizes the evidence on the comparative clinical effectiveness of standard screening mammography and supplemental imaging to screen women with mammographically dense breasts following a negative mammogram. As of 2015, 24 states in the United States have passed legislation requiring mammography facilities to notify women with dense breasts about their density. For example, on April 1, 2013 a law went into effect in California requiring mammography facilities to inform women with dense breasts about the potential for “masking” and the increased risk of breast cancer associated with dense breast tissue. Masking occurs when breast cancers are hidden by dense breast tissue on a mammogram, which, like cancer, appears white. The law requires the following language be included in reports sent to women who have dense breast tissue:
Your mammogram shows that your breast tissue is dense. Dense breast tissue is common and is not abnormal. However, dense breast tissue can make it harder to evaluate the results of your mammogram and may also be associated with an increased risk of breast cancer. This information about the results of your mammogram is given to you to raise your awareness and to inform your conversations with your doctor. Together, you can decide which screening options are right for you. A report of your results was sent to your physician.
The primary motivation for the law is to alert women to the masking effect of dense breast tissue on their mammograms. However, dense breast tissue may also play an important role by identifying women at high enough risk for breast cancer to warrant additional imaging with a different technology. A number of new technologies and new applications of existing technologies have been promoted to enhance screening in women with dense breasts. These include hand-held and automated breast ultrasound, magnetic resonance imaging (MRI), and digital breast tomosynthesis (DBT).
This chapter will summarize the evidence about film and digital screening mammography in women with dense breasts and the harms and benefits of supplemental screening after a normal screening mammography examination. We will also assess ways to estimate the overall risk of breast cancer for women with dense breasts and a negative mammogram to guide the decision about which women are more likely, and which less, to benefit from supplemental screening.
Background
Breast Cancer
Breast cancer is the most common cancer in women worldwide. There were approximately 1.7 million new cases of breast cancer and 552,000 deaths from breast cancer in 2012. In the United States, mortality from breast cancer has declined by about 2.2% per year since 1990, a 28% overall decline. The median values from a series of models estimated that a little more than half of the decline was due to improvements in therapy for breast cancer and that a little less than half (46%) was due to early diagnosis from mammography while a more recent study in Norway found only 33% of the reduction in breast cancer mortality was due to screening. An analysis of 30 years of data from the United States Surveillance, Epidemiology, and End Results (SEER) data also called into question the contributions of screening mammography to decreasing breast cancer mortality. Bleyer and Welch estimated that 31% of breast cancer diagnosed with mammography represents “overdiagnosis” (ie, identification of cancers unlikely to cause significant morbidity or mortality) and concluded that screening mammography has had, at best, only a small effect on breast cancer mortality.
Screening for Breast Cancer
The primary method worldwide used to screen for breast cancer is mammography. Nine large clinical trials established the efficacy of screening mammography by randomizing over 600,000 women and following them for 10–20 years. The results have been summarized in many systematic reviews and meta-analyses. There is general consensus that, for women between the ages of 50 and 69 years, screening mammography reduces breast cancer mortality by approximately 20% to 25% after 15 years of follow-up. For average-risk women between the ages of 40 and 49 years, there remains controversy about whether the benefits of routine mammography outweigh the harms, such that most countries that offer mammography do not offer routine screening to women aged 40–49 years and some recommend a discussion of the benefits and harms of mammography allowing women to decide on screening based on their personal preference.
Digital Mammography
Mammography was traditionally performed with film. It was one of the last radiographic procedures to transition from film to digital imaging because mammography requires extremely high resolution to be effective. Digital image acquisition improves the signal to noise ratio of X-ray detection over a wider contrast range than film. Digital enhancement of the images at computer workstations may also improve the accuracy of mammographic interpretation. In particular, increased contrast resolution improves the detection of low contrast lesions in radiographically dense breasts. Digital mammography has become the standard across the United States. As of October 1, 2015, 97.5% (14,769/15,153) of all US mammography machines accredited by the Food and Drug Administration (FDA) are full-field digital.
Mammographic Breast Density
Mammographic density refers to areas within the breast that absorb significant amounts of X-ray energy and show up as relatively white areas on the mammogram. These correspond to regions in the breast that are rich in epithelial and stromal tissue while the nondense (darker gray areas) correspond to regions that are predominantly fat.
In the United States, the Breast Imaging Reporting and Data System (BI-RADS) of the American College of Radiology classifies density in the following four breast composition categories:
- a.
The breasts are almost entirely fatty.
- b.
There are scattered areas of fibroglandular density.
- c.
The breasts are heterogeneously dense, which may obscure small masses.
- d.
The breasts are extremely dense, which lowers the sensitivity of mammography.
The prevalence of high breast density (BD; heterogeneous or extremely dense tissue) is 43% in women 40–74 years old, which represents almost 28 million women in the United States. The prevalence varies by age declining from 57% for women between the ages of 40 and 44 years to 28% for women ages 85 years and older. High BD using the BI-RADS categories is also more common in Asian women.
It has been known for years that the sensitivity of film mammography is lower in women with dense breasts than in women with fatty breasts. There is a masking effect due to mammographic density. In the Breast Cancer Surveillance Consortium (BCSC), a US collaboration of breast imaging registries, the sensitivity of film mammography decreased markedly with increasing density (see Table 10.1 ). This study evaluated the results from 463,372 screening film mammograms performed between 1996 and 1998. Among women in the low density categories, the sensitivity of mammography was 88% and 82% for density categories a and b, respectively, but this decreased to 69% for women with heterogeneously dense breasts and to 62% for women with extremely dense breasts.
| Study | Type | BI-RADS Density Category | |||
|---|---|---|---|---|---|
| Almost Entirely Fatty (a) | Scattered Fibroglandular Densities (b) | Heterogeneously Dense (c) | Extremely Dense (d) | ||
| BCSC (Carney 2003) | Film | 88.2 | 82.1 | 68.9 | 62.2 |
| DMIST (Pisano 2005) | Film | 55 a | |||
| Digital | 70 a | ||||
| BCSC (Kerlikowske 2011) | Film | 85.7 | 85.1 | 79.3 | 68.1 |
| Digital | 78.3 | 86.6 | 82.1 | 83.6 | |
a The DMIST study reported results for the combined high-density categories only.
BD and Digital Mammography
As described above, the increased contrast resolution of digital mammography improves the detection of low contrast lesions in radiographically dense breasts. Thus digital mammography should improve the sensitivity of mammography in women with dense breast tissue compared to film.
The Digital Mammography Imaging Screening Trial (DMIST) study is the largest trial directly comparing digital mammography to plain film mammography ( n = 42,760). All women were screened with both film and digital mammography on the same visit. Mammograms were read independently by radiologists blinded to the results of the other mammogram. In DMIST, digital mammography had the same recall and biopsy rates as film mammography. Digital mammography was more sensitive than film for younger women with denser breasts (59.1% vs 27.3%, p = 0. 0013). Among women of all ages with either heterogeneously dense or extremely dense breasts, digital mammography was also more sensitive than film mammography (70% vs 55%, p = 0.02, Table 10.2 ). Similarly, in women with dense breast tissue there was a trend towards greater specificity with digital mammography (91% vs 90%, p = 0.09) and the overall accuracy of digital mammography, as measured by the area under the receiver operator curve, was greater than that of film mammography (0.78 vs 0.68, p = 0.003).
| Study | Type | BI-RADS Density Category | |||
|---|---|---|---|---|---|
| Almost Entirely Fatty (a) | Scattered Fibroglandular Densities (b) | Heterogeneously Dense (c) | Extremely Dense (d) | ||
| BCSC (Kerlikowske 2011) | Rate a | 1.8 | 3.3 | 4.8 | 5.1 |
| Sens | 78 | 87 | 82 | 84 | |
| Spec | 95 | 91 | 87 | 89 | |
a Rate =breast cancer detection rate per 1000 women screened.
The BCSC has reported on the accuracy of screening mammography based on a comparison of 231,034 digital mammograms and 638,252 film mammograms performed between January 1, 2000 and December 31, 2006. Similar to the prior study, the sensitivity of film mammography decreased from 86% to 68% across the four BD categories (see Table 10.2 ). However, for digital mammography, the sensitivity of digital mammography remained greater than 80% for the highest density categories and did not appear to decrease with increasing density (see Table 10.2 ). As in the DMIST trial, digital mammography was significantly more sensitive than film mammography in women with dense breasts, but had lower specificity.
Table 10.2 shows the cancer detection rate and specificity in addition to the sensitivity of digital mammography by BI-RADS density category in the BCSC study. Despite concerns about the test performance of mammography in dense breasts, more breast cancers are found per 1000 digital screening mammograms in the dense breast categories than in the nondense categories. This highlights the general principle that the yield of screening tests is greater as the underlying risk of the population screened goes up. Women with dense breasts are at higher risk, so the cancer detection rate is higher. These data also suggest that the masking effect of BD is reduced when digital mammography is used.
Table 10.3 summarizes important outcomes with film and digital mammograms in the three largest studies that report data on both digital and film mammography. These are useful benchmarks to use when evaluating the potential yield of additional imaging compared to no additional imaging. The biopsy rate and cancer detection rate did not differ between women screened with digital or film mammography in any of these studies, although the recall rate for digital was higher in the BCSC (100 vs 93 per 1000, p < 0.001). When cancer detection was stratified by BD in the BCSC, no statistical differences were found between digital and film mammography. However, there was a nominal trend toward higher cancer detection in women with extremely dense breasts (5.1 vs 3.8 per 1000, p = 0.17); the authors concluded that this was primarily due to better detection in women aged 40–49 with extremely dense breasts.
| Study | Type | Mammograms, n | Recall Rate per 1000 | Biopsy Rate per 1000 | Cancer Detection per 1000 | PPV3 |
|---|---|---|---|---|---|---|
| DMIST (Pisano 2005) | Film | 42,555 | 86 | 16.0 | 4.1 | 24.4 |
| Digital | 42,555 | 86 | 15.9 | 4.4 | 26.0 | |
| Vestfold (Vigeland 2008) | Film | 324,763 | 42 | NR | 6.5 | 15.1 |
| Digital | 18,239 | 41 | NR | 7.7 | 18.5 | |
| BCSC (Kerlikowske 2011) | Film | 638,252 | 93 | 10.6 | 3.8 | 24.7 |
| Digital | 231,034 | 100 | 11.0 | 3.8 | 25.3 |
It is worth noting in Table 10.3 that in Europe, the recall rate for mammography is generally about half that observed in the United States. Thus, one of the harms of mammography, recalls for false-positive imaging results, is less common in Europe. It is important to keep this in mind when evaluating how to apply the results from studies of supplemental screening performed in Europe versus the United States to different countries performing mammography.
In summary, the findings from both the DMIST and BCSC studies, along with the results from other high-quality studies, highlight a critical difference between digital and film mammography in women with dense breasts. The studies find that digital mammography is more sensitive than film mammography in women with dense breasts. Therefore, the masking effect of BD observed with film mammography is reduced, but not eliminated with digital mammography.
Supplemental Screening Modalities for Breast Cancer Screening
There are many imaging approaches to screen for breast cancer in addition to mammography. Breast MRI has been increasing since the American Cancer Society recommended the use of MRI to screen women at highest risk for breast cancer in 2007, based primarily on genetic susceptibility. Hand-held ultrasound has been used as a diagnostic tool to evaluate women with breast masses and has been promoted by some as a screening tool. The FDA has approved automated whole breast ultrasound, which scans and records ultrasound images of the entire breast, for breast cancer screening. Finally, DBT, a three-dimensional (3D) extension of digital mammography, has been viewed as holding significant promise in breast cancer screening. Other imaging modalities, such as contrast-enhanced mammography, thermography, diffuse optical tomography, sestamibi, positron emission mammography, dedicated breast computed tomography, electrical impedance scanning, MRI spectroscopy, and breast-specific gamma imaging are still in early investigational phases and will not be considered further.
All four of the advanced imaging technologies considered in this chapter produce multiple 2D images of the breast. This is particularly relevant in mammographically dense breasts because breast cancers may be obscured by superimposed dense tissue.
Hand-Held ULTRASOUND of the Breast
Hand-held ultrasound (HHUS) is widely used at breast imaging centers to evaluate breast masses and to guide both cyst aspiration and percutaneous breast biopsy procedures. It is particularly useful to differentiate fluid filled cysts from solid masses (cysts are rarely cancerous). Over time, HHUS has evolved to use higher frequency sound waves to generate images of the breast with improved resolution. In addition, earlier generations of HHUS were not able to penetrate deeply into breast tissue and had a limited field of view. Advantages of ultrasound include its widespread availability and the ability to evaluate tissue that is dense on mammography without additional ionizing radiation, which can potentially increase the risk for future cancers. It is also perceived to be more comfortable than mammography because it does not require compression of the breasts.
Ultrasound also has limitations. The primary concern with HHUS is the high number of false-positive findings, which often lead to unnecessary biopsies. There are also concerns about the operator dependency and reproducibility of the examinations. Like MRI, HHUS takes time. The average length of time for breast HHUS imaging in a recent study was 19 minutes. In that study and many others, a breast radiologist performed the ultrasound, but technologists are also performing screening ultrasounds. At a minimum, the breast radiologist needs to be available to review static images saved by the performing technologist in real time so that additional images can be acquired if necessary.
Automated Whole Breast Ultrasound
Automated whole breast ultrasound (ABUS) uses computer driven ultrasound transducers to scan the entire breast under the guidance of a technician. A technician compresses the woman’s breasts to her chest wall and applies ultrasound gel. A breast-shaped transducer is placed on the compressed breast and automatically scans the entire breast. The entire procedure, including patient preparation, takes about 15 minutes to complete. ABUS reduces the need for radiologists to perform the scan and decreases the length of time of the exam, thus addressing two of the shortcomings of HHUS. It also produces a scan that should have less operator dependence. The radiologist can review the scan independently using software that displays the images individually or sequentially in a movie mode. The primary drawbacks to ABUS are the high number of false-positive examinations, inability to image very large breasts, the storage requirements for the data acquired during the scan, and the time required to read the images.
Breast MRI
MRI uses strong magnetic fields to image the breast, rather than ionizing radiation. The system uses computational algorithms to generate detailed cross-sectional views of the breast. Mammography requires repositioning of the breast for each desired view. In contrast, the MRI examination is typically performed with the woman in the prone position lying on a platform placed in the MR chamber that allows the breast to extend dependently from the woman and does not require repositioning. A contrast agent, gadolinium, is injected through an intravenous catheter (IV) to improve the images of the breast.
In studies of high-risk women, MRI approximately doubles the number of breast cancers that are detected compared to film mammography or breast ultrasound. However, several factors limit the widespread use of MRI for screening. These include an increase in false-positive test results, the need for placement of an intravenous catheter to infuse contrast, the length of time required for the examination, the cost of the examination, limited availability of breast MRI facilities (with special breast-specific magnetic coils and biopsy capability), and contraindications to the use of MRI due to claustrophobia, pacemakers, and other metallic implants. In addition, mammography has been found to be more sensitive than MRI for the detection of ductal carcinoma in situ, a noninvasive cell abnormality in the milk ducts, and some invasive breast cancers, so the two are typically used together.
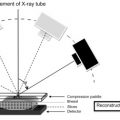
Digital Breast Tomosynthesis
DBT uses a conventional X-ray source that sweeps along an arc around the breast to acquire multiple 2D digital images. Breast compression is performed using the same device and technique as conventional digital mammography. The procedure to obtain each digital view is complete in less than 20 s. One of the advantages of DBT is that the images can be acquired immediately following the digital mammogram. Like MRI, computational algorithms synthesize the resulting 2D digital images to create tomograms (ie, slices) allowing for a 3D reconstruction of the breast. The tomograms can be displayed individually (similar to enhanced conventional mammograms) or in a dynamic movie mode.
There are several drawbacks to DBT. The dose of ionizing radiation for DBT is about the same as or a little higher than that used for a conventional digital mammography. Currently, a standard digital image is also acquired, so the total dose is at least twice that of digital mammography alone. The technology and algorithms used for DBT are still in evolution. One of the crucial areas is the development of techniques to biopsy lesions that are only seen on DBT. DBT can also be used to generate a virtual 2D digital mammogram (referred to as synthetic 2D mammogram), which could eliminate the need for performing digital mammography and thus eliminate the excess ionizing radiation. This technology has been developed and approved by the FDA for clinical use, has not been used in studies cited in this chapter (see chapter “Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis” in this book for evidence on DBT with synthetic 2D mammography). Finally, the reading time for DBT is about twice that required for digital mammography.
Screening Hand-Held Breast Ultrasound (HHUS) Studies in Women With Dense Breasts
HHUS is the most studied supplemental screening technology for women with dense breasts ( n > 80,000). In general, women in these studies underwent mammography first and those with negative mammogram results were subsequently screened by HHUS.
Japan Strategic Anticancer Randomized Trial
In Japan, investigators in the Japan Strategic Anticancer Randomized Trial (J-START) trial randomized 72,998 asymptomatic woman women ages 40–49 years to mammography plus HHUS or mammography alone twice over a 2-year period. All women also underwent clinical breast examination. In the first round of screening, the cancer detection rate with the combination of mammography and HHUS was higher than that of mammography alone (5.0 per 1000 vs 3.3 per 1000, p = 0.003). As expected, the sensitivity was higher in the group receiving both tests (91.1% vs 77.0%, p = 0.004) and the specificity was lower (87.7% vs 91.4%, p < 0.0001). Fewer interval cancers were detected in the dual screened arm of the study (0.05% vs 0.10, p = 0.034). The recall rate of the HHUS group was higher than that of the mammography group (12.6% vs 8.8%) as was the biopsy rate (4.5% vs 1.8%). The study does not directly address the question of the added value of HHUS in women with dense breasts because the study screened all women ages 40–49 years. However young Asian women have a very high prevalence of dense breasts, so the results are a reasonable approximation.
American College of Radiology Imaging Network 6666 Trial
The American College of Radiology Imaging Network (ACRIN) 6666 trial is the only US prospective clinical trial of HHUS with follow-up for multiple screening rounds. The population studied was higher risk than that of a typical screening population, so the biopsy rate, cancer detection rate, and positive predictive values will be higher than those of a screening population. For instance, in the first round the biopsy rate based on mammography was 14.4 per 1000 examinations, the cancer detection rate was 7.5 per 1000 examinations, and the PPV3, which represents the percentage of biopsies that are positive for cancer, was 31%, all of which are higher than expected for mammography in a screening population (10 per 1000, 5 per 1000, 25%, respectively).
The ACRIN 6666 trial recruited 2809 high-risk women to receive both mammography (film or digital) and ultrasound in a randomized order. High-risk was defined by at least one of the following: a personal history of breast cancer; positive for BRCA1 or BRCA2 mutation; a lifetime risk ≥25%, a 5-year risk ≥2.5% or ≥1.7% with extremely dense breast tissue; prior biopsy with atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ or atypical papilloma; or prior mantle radiation. The study also required that the women have at least one quadrant of one breast with heterogeneously dense or extremely dense tissue on a prior mammogram.
In the first screening round, mammography detected 20 cancers (cancer detection rate 7.6 per 1000 examinations) and ultrasound detected an additional 14 cancers (5.9 per 1000 examinations). There were two interval cancers so the sensitivity of mammography was 55.6% (20/36) and the sensitivity of ultrasound in women with negative mammograms was 87.5% (14/16). The number of recalls increased from 306 with mammography alone to 707 with mammography plus ultrasound, a 2.3-fold increase in the recall rate (from 115.1 per 1000 examinations to 265.9 per 1000). The number of breast biopsies increased from 65 to 272, a 4.2-fold increase (from 24.4 per 1000 examinations to 102.3 per 1000). The PPV3 for ultrasound in women with negative mammograms was only 6.8%.
In round 3, women were offered MRI in addition to HHUS and mammography. The 612 women in the MRI substudy had higher risk for breast cancer and were younger than those who declined participation. In this group of participants, mammography alone detected five cancers, ultrasound detected an additional two cancers (sensitivity for the combination 43.8%, cancer detection rate 11.4 per 1000 examinations) and MRI detected nine additional cancers (sensitivity 100%, incremental cancer detection rate 14.7 per 1000 examinations and combined cancer detection rate 26.1 per 1000 examinations). The nine cancers detected by MRI only were small (median 8.5 mm) and all were lymph node negative. Both cancers seen only with HHUS (not mammography) were also diagnosed with MRI. The high cancer detection rate in the women in the MRI group reflects the high underlying risk for cancer in the women who agreed to participate in the substudy. The recall rate was 85.0 per 1000 examinations for mammography alone, 163.4 per 1000 for the combination of mammography plus HHUS, and 260.0 per 1000 for MRI. The biopsy rate was 62.1 per 1000 examinations for the combination of mammography plus HHUS and 132.3 per 1000 for the combination with MRI. The PPV3 for MRI in women with a negative mammogram was 22.4%, which is much higher than that of ultrasound.
In this high-risk population, supplemental screening with HHUS produced a relatively high yield of cancers the first round of screening, approximately doubling the cancer detection rate, but this decreased with subsequent rounds. In order to find the additional cancers, the recall rate more than doubled so that one in four women (26.6%) were recalled in the first round. The number of biopsies performed increased by a factor of 4. In the first round, the combination of ultrasound plus mammography led to almost as many biopsies (10.2% of women) as women recalled with mammography alone (11.5% of women). The addition of MRI more than doubled the cancer detection rate of mammography plus ultrasound, but was associated with an even higher recall rate and a doubling of the biopsy rate. The PPV3 for ultrasound in women with negative mammograms was very low (6.8% round 1, 7.1% rounds 2 and 3) compared to mammography alone (29.1% round 1, 38.1% rounds 2 and 3). The PPV3 for MRI in women with negative mammograms was 22.4%.
Observational Studies
We reviewed 15 observational studies on HHUS. The participants in these studies had a mean age usually in the 50s with a broad range (25–91 years). Most included asymptomatic women presenting for screening mammography who were found to have dense breasts, although the definition of high BD varied somewhat. The majority of the studies were done outside of the United States. Four retrospective cohorts described the findings in Connecticut, the first state in the United States requiring mandatory BD notification. These studies represent the best evidence in the US population for the incremental cancer detection rate with HHUS in average-risk women with dense breasts, although they do not have data on interval cancer rate. Two other studies in the United States reported results from imaging performed in the year 2000 and earlier. A radiologist performed the HHUS in the majority of the studies. Nine of the studies reported no follow-up of participants. This means that the sensitivity, specificity, and negative predictive value reported from those studies will overestimate the true values.
The biopsy rate for women having HHUS after normal mammography ranged from 12–114 per 1000 examinations with a median of 46, which is threefold higher than with mammography. There was also a wide range of estimates across the studies for the recall rate (2.0% to 11.7%, median 6.5%) and the PPV3 (3.2% to 18.4%, median 7.1%). The heterogeneity of these results was likely due to a combination of factors. These include the study design (prospective, retrospective), the use of film or digital mammography, differences in the assessment of mammography across countries, whether a radiologist or a technician performed the HHUS, the level of experience and training of the person performing the HHUS, and differences in the populations studied (age distribution, breast cancer risk factors, time since last mammogram).
Most of the cancers detected by HHUS after negative mammography are small, node negative, early stage cancers. These are the cancers that are potentially curable by early detection before they potentially develop into cancers with a poorer prognosis. Cancers at an early stage also require less aggressive therapy: the patient may be eligible for lumpectomy rather than mastectomy and may not require systemic chemotherapy. Thus early detection may improve both quality and quantity of life. On the other hand, many of these early stage cancers may not have progressed before the next routine screening examination with mammography. Thus, they may ultimately have been detected and cured with mammographic screening alone. In addition, some proportion of these cancers may represent overdiagnosis: the identification of a cancer that would not have ever progressed to cause symptoms prior to the death of that individual woman (see chapter “Challenges in Understanding and Quantifying Over-Diagnosis and Over-Treatment” for more information on overdiagnosis). The identification of such cancers would lead to unnecessary labeling of the woman as someone who has cancer as well as unnecessary surgery and chemotherapy (ie, overtreatment). The only way to test which of these two competing hypotheses is true would be to perform a randomized trial comparing the two approaches to breast cancer screening.
Summary: Screening HHUS of the Breast
There are no studies evaluating the impact of adding HHUS to mammographic screening among women with dense breast tissue that address the key patient-centered outcomes of breast cancer mortality and disease-free survival. The available body of evidence, focusing largely on shorter-term outcomes of recall, biopsy, cancer detection, and false-positive rates, is limited by multiple factors. There were a large number of studies, but the heterogeneity of the study designs, populations, and results preclude the use of meta-analytic techniques to combine the results. The majority of the studies had a negative film-screen mammography examination followed by supplemental screening ultrasound, were retrospective, did not fully report the recall rate, and were not able to calculate sensitivity because women with negative mammograms were not followed for interval cancer. The best estimates for sensitivity and specificity in the United States come from the ACRIN 6666 trial (87.5% and 81.9%, respectively) because it was a well-conducted prospective trial. The incremental cancer detection rate from the four studies on the Connecticut experience was approximately 2 additional cancers per 1000. The results from Connecticut are more likely to be representative of routine clinical practice in the United States and to represent average-risk women with dense breasts. Table 10.4 summarizes the key statistics from the six with direct evidence and the ACRIN 666 study (high quality indirect evidence).