It is thought that myeloproliferative neoplasms (MPNs) are driven by somatic mutations, although hereditary factors also play a prominent role in the pathogenesis of the disease. Hereditary thrombocytosis and erythrocytosis are not malignant disorders but are clinically similar to MPNs. Several mutations have been found that explain a proportion of hereditary thrombocytosis and hereditary erythrocytosis. Germline variants can influence the risk of leukemic transformation in MPNs and the course of the disease through interaction with acquired chromosomal aberrations. Overall, it has been shown that germline factors play an important part in MPN pathogenesis.
- •
Myeloproliferative neoplasms (MPNs) are mainly driven by somatically acquired point mutations and chromosomal aberrations.
- •
Germline factors can predispose to the development of MPN, acquisition of somatic mutations, and chromosomal aberrations, as well as modify the clinical course of the disease.
- •
Familial clustering of MPN in 5% to 10% of cases, increased risk of the disease in relatives of patients with MPN, and the existence of biclonal MPN provide evidence of germline MPN susceptibility.
- •
Hereditary thrombocytosis and erythrocytosis have similar clinical symptoms as MPN; however, these disorders show distinct features, such as polyclonal hematopoiesis, single lineage involvement, and absence of disease progression.
- •
Germline mutations in JAK2 , MPL , and THPO cause hereditary thrombocytosis, whereas germline mutations in EPOR , oxygen-sensing pathway genes, or genes affecting oxygen affinity of hemoglobin result in hereditary erythrocytosis.
- •
The common JAK2 GGCC haplotype predisposes to the development of JAK2-postive MPN.
- •
A nonsynonymous germline variant in the ERCC2 ( XPD ) gene increases the risk of leukemic transformation and the development of new primary tumors in patients with MPN.
- •
Rare germline variants in the regions of loss of heterozygosity can have an influence on MPN pathogenesis.
- •
The germline mutations responsible for the familial cases of MPN have not been identified so far.
Introduction
Myeloproliferative neoplasms (MPNs) are chronic hematological malignancies of clonal origin characterized by the predominant involvement of myeloid lineages, accumulation of terminally differentiated blood cells, and the tendency to transform to secondary acute myeloid leukemia (sAML). MPNs are a heterogeneous group of disorders and include 9 disease entities according to the 2008 World Health Organization classification. The classic MPN or Ph-chromosome-negative MPN include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The MPN disease subtypes exhibit specific phenotypic features but share many clinical and molecular features. MPNs are characterized by high numbers of differentiated cells of myeloid origin in peripheral blood, mainly red blood cells in PV or platelets in ET. Patients with PMF usually have a lower number of myeloid cells as a result of bone marrow fibrosis, and consequent extramedullary hematopoiesis often manifests with splenomegaly. PV and ET may progress into secondary myelofibrosis, which has a similar clinical presentation as PMF but a much higher rate of transformation to sAML. Patients with MPN frequently have thrombotic or hemorrhagic complications; in some cases those complications are the first presentations of the disease. Typically, MPNs are diseases of the elderly; the age of onset is around 50 to 60 years, although cases in younger ages are also observed, especially in the presence of familial history. The life expectancy of patients with PV and ET is more than a decade. In PMF, the life expectancy is 3 to 5 years on average, whereas after the transformation to sAML, the median survival is a few months with no effective treatment available. The treatment in MPN is directed toward controlling the symptoms and the progression of the disease; it proves to be enough in most cases.
Role of somatic mutations in MPN
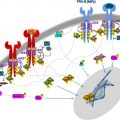
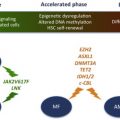
Acquired genetic changes drive clonal progression in MPN, as in other cancers. Several recurrent chromosomal aberrations and point mutations have been identified in the pathogenesis of MPN, with variable frequency in PV, ET, and PMF. The most common somatic mutation in MPN is the V617F mutation in the Janus kinase 2 ( JAK2 ) gene, observed in about 95% of patients with PV and 50% to 60% in ET and PMF. The JAK2-V617F mutation is often associated with acquired uniparental disomy on the short arm of chromosome 9 (9pUPD), which makes the mutation homozygous particularly in PV and PMF. Mutations in exon 12 of JAK2 are present in 1% to 3% of PV cases. Somatic activating mutations in the thrombopoietin receptor gene ( MPL ) are detected in 1% to 5% of the cases of PMF and ET, predominantly in a mutually exclusive manner with JAK2 mutations. Other mutations commonly found in patients with MPN are in TET2 , CBL , EZH2 , and ASXL1 , with variable frequency in 3 MPN subtypes ( Fig. 1 ). Another group of mutations are acquired during the disease progression and transformation to sAML, such as TP53 , RUNX1 , NPM1 , FLT3 , IDH1 , and IDH2 . Several recurrent chromosomal aberrations have been described in patients with MPN. Deletions on chromosome 13q, 20q, 12p, uniparental disomies (UPDs) of 9p, 1p, 11q, 7q, trisomy of chromosome 8 and 9, and gains of chromosome 9p are often observed in the chronic phase of the disease, whereas deletions of 5q, 7q, UPDs of 17p, and gains of chromosome 1q and 3q are mostly present in advanced disease and at leukemic transformation. This remarkable diversity of somatic mutations and chromosomal aberrations in MPN contributes to the genetic complexity of the disease, similar to other forms of cancer. The variability in mutation frequencies in 3 subtypes of MPN partially explains the clinical differences among PV, ET, and PMF. However, a significant part of the phenotypic diversity is yet unexplained.
Role of somatic mutations in MPN
Acquired genetic changes drive clonal progression in MPN, as in other cancers. Several recurrent chromosomal aberrations and point mutations have been identified in the pathogenesis of MPN, with variable frequency in PV, ET, and PMF. The most common somatic mutation in MPN is the V617F mutation in the Janus kinase 2 ( JAK2 ) gene, observed in about 95% of patients with PV and 50% to 60% in ET and PMF. The JAK2-V617F mutation is often associated with acquired uniparental disomy on the short arm of chromosome 9 (9pUPD), which makes the mutation homozygous particularly in PV and PMF. Mutations in exon 12 of JAK2 are present in 1% to 3% of PV cases. Somatic activating mutations in the thrombopoietin receptor gene ( MPL ) are detected in 1% to 5% of the cases of PMF and ET, predominantly in a mutually exclusive manner with JAK2 mutations. Other mutations commonly found in patients with MPN are in TET2 , CBL , EZH2 , and ASXL1 , with variable frequency in 3 MPN subtypes ( Fig. 1 ). Another group of mutations are acquired during the disease progression and transformation to sAML, such as TP53 , RUNX1 , NPM1 , FLT3 , IDH1 , and IDH2 . Several recurrent chromosomal aberrations have been described in patients with MPN. Deletions on chromosome 13q, 20q, 12p, uniparental disomies (UPDs) of 9p, 1p, 11q, 7q, trisomy of chromosome 8 and 9, and gains of chromosome 9p are often observed in the chronic phase of the disease, whereas deletions of 5q, 7q, UPDs of 17p, and gains of chromosome 1q and 3q are mostly present in advanced disease and at leukemic transformation. This remarkable diversity of somatic mutations and chromosomal aberrations in MPN contributes to the genetic complexity of the disease, similar to other forms of cancer. The variability in mutation frequencies in 3 subtypes of MPN partially explains the clinical differences among PV, ET, and PMF. However, a significant part of the phenotypic diversity is yet unexplained.
Predisposition to MPN
Although MPNs are mainly driven by somatically acquired point mutations and chromosomal aberrations, germline factors have been shown to play a role in MPN pathogenesis. There are several lines of evidence that point out the importance of germline genetics in MPN. The phenotypic diversity of MPN (ie, the existence of 3 clinically different disease entities with similar mutational profile) is not properly explained by somatic defects and environmental factors. Thus, hereditary factors have been suggested to play a role in influencing the disease phenotype and resulting in the development of PV, ET, or PMF in the presence of the same mutations, most notably JAK2-V617F. There have been some reports of the existence of biclonal MPN when 2 independent malignant clones develop in the same patient. The probability of occurrence of such an event is extremely low, and the only explanation of that phenomenon could be the existence of hereditary predisposition in such patients. Surprisingly high number of patients with MPN have close relatives with MPN. Common polymorphisms also have influence of predisposing to MPN, exemplified by the discovery of an MPN risk haplotype spanning JAK2 gene.
GGCC haplotype
In 2008, a study investigated the influence of germline single nucleotide polymorphisms (SNP) in several candidate genes on the clinical phenotype of the disease, particularly the difference between PV and ET. They found that several SNPs in the region of the JAK2 gene are significantly different in PV compared with ET. Two other groups followed up this discovery and refined the association, showing that a common haplotype (46/1 or GGCC) in the JAK2 locus predisposes to JAK2-V617F-positive MPN. The difference of haplotype frequency is caused by a different proportion of patients with PV and ET that carried the JAK2 mutation. In parallel, Olcaydu and colleagues (2009) investigated the multiple acquisition of JAK2-V617F in MPN. Studying the patients who were V617F positive that were heterozygous for the common haplotype on JAK2 , denoted as GGCC, the mutation was found to be gained significantly more often on one of the alleles. Following up on this finding, Olcaydu and colleagues (2009) came to the same conclusion that the GGCC haplotype predisposes to V617F-postive MPN. Later it was demonstrated that the same haplotype also predisposes to the JAK2-exon12 mutation-positive MPN and MPL-positive MPN. Moreover, it was shown that the JAK2 haplotype is a susceptibility factor for ET and PMF independent of JAK2 status.
Overall, the GGCC haplotype seems to be one of the major germline factors involved in the pathogenesis of MPN. The association of the haplotype with JAK2-V617F-positive MPN is one of the strongest reported so far, with an odds ratio around 2.5. Most of the SNP associations for other diseases have odds ratios less than 1.5. In the era of genome-wide association studies, it is remarkable that JAK2 haplotype association was found by other approaches, underlining its strong susceptibility. So far, no other common SNP has been reported to be associated with MPN. Although there might be more SNPs with high frequency in populations that predispose to MPN, it is highly unlikely that there will be another one of similar strength as the JAK2 haplotype. The JAK2 haplotype explains a major proportion of MPN heritability, which is more than 50% of the increased risk in the relatives of patients with MPN. The population attributable risk percent has been estimated to be from 28% to 46%. Thus, the JAK2 GGCC haplotype is the major common genetic susceptibility factor for MPN.
Familial clustering of MPN
As soon as cellular and molecular markers became available to separate MPN from other hereditary conditions, such as familial erythrocytosis, several distinct pedigrees with true clonal MPN have been described. Subsequent reports have shown frequent familial clustering of MPN, with an estimated 5% to 10% of MPN having familial history and a several-fold increase of MPN risk in the relatives of patients with MPN. Familial cases of MPN are defined as 2 or more cases among relatives. However, it must be taken into account that 2 cases of sporadic MPN can also occur in a family by chance within populations of many millions of people.
So far, the causative germline mutations have only been found in a small proportion of familial MPN. For the rest of the families, the underlying genetic cause remains unknown. The germline mutations involved in familial MPN have to confer much higher risk than the GGCC haplotype, which increases the risk twofold to threefold. The estimated average penetrance level of MPN mutations is around 30% ; however, the penetrance varies significantly among individual families. Some reports have shown disease anticipation in familial MPN when in each following generation the age of onset of the disease is lower. However, others have not confirmed this phenomenon. Familial clustering of MPN is one of the most convincing evidences that germline factors play an important role in MPN pathogenesis.
Clinical and genetic heterogeneity in familial MPN
The MPN pedigree structures published to date show clear heterogeneity. Most of the families show the autosomal dominant pattern of inheritance, although in some other cases autosomal recessive inheritance cannot be excluded. The familial MPN in most cases display low penetrance ; however, there are pedigrees with high, almost complete penetrance. The age of onset in familial cases of MPN is lower than in sporadic MPN, but there is evidence of variability.
The MPN families also differ according to MPN subtype. There are families whereby all the affected members have the same subtype of the disease (eg, ET, PV, or PMF), whereas in other families the affected members have different diagnoses. In addition, some families have an unusually high tendency to transform to sAML. This myriad of MPN family types points toward extreme genetic heterogeneity and supports the idea that there might be many different germline mutations that all cause MPN.
Perhaps the most intriguing variability in familial MPN cases is the somatic acquisition of JAK2 , MPL , and TET2 mutations in a subpopulation of familial cases. This variability leads to the hypothesis that familial MPN may be comprised of 2 subgroups of fundamentally different diseases. The first group, true familial MPN, comprises the families whereby the underlying germline defect does not itself drive the disease but predisposes to the acquisition of oncogenic mutations, like JAK2-V617F. These pedigrees are predicted to have lower penetrance and a later age of onset because the disease initiation depends on the acquisition of a somatic mutation. There have been several familial linkage studies performed on families with MPN and most of the candidate genes frequently mutated somatically have been ruled out. The second group, MPN-like disorders, is discussed later.
Hereditary erythrocytosis and thrombocytosis
In a proportion of pedigrees, the germline mutation itself is causing the disease. These MPN-like families have higher penetrance of the disease, an earlier age of onset, and do not carry somatic JAK2 mutations. From another point of view, these families might not be considered true familial MPN but rather forms of hereditary thrombocytosis or erythrocytosis. It is important to differentiate true familial MPN from the cases of hereditary erythrocytosis and thrombocytosis, which can have clinical manifestations somewhat similar to PV and ET, respectively. There are several differences between these two disorders. Hereditary thrombocytosis and erythrocytosis are characterized by polyclonal hematopoiesis, involvement of single myeloid lineage, high penetrance, and absence of progression of the disease. By contrast, in patients with familial MPN, hematopoiesis is clonal, usually involves multiple lineages, and the disease frequently progresses to secondary myelofibrosis and sAML. Progenitor cells from patients with familial MPN form spontaneous erythroid colonies, a feature absent in healthy people or hereditary erythrocytosis.
In many cases of hereditary erythrocytosis and thrombocytosis, the underlying germline mutation is known. Mutations in oxygen-sensing pathway genes ( VHL, EGLN1 , and so forth) or genes affecting oxygen affinity of hemoglobin result in hereditary secondary erythrocytosis as a consequence of high erythropoietin levels. EPOR truncating mutations cause primary familial and congenital polycythemia in which the erythrocytosis is primary as a result of the removal of inhibitory domain by the truncations and hypersensitivity to erythropoietin. Hereditary thrombocytosis is caused by mutations in the thrombopoietin gene ( THPO ), which explains only a part of those cases.
There is another group of families that have the MPN-like phenotype but also share some characteristics of hereditary thrombocytosis. These cases are caused by germline mutations in MPL and JAK2 genes and are discussed in the following section.
Germline MPL mutations
A germline mutation in the thrombopoietin receptor gene ( MPL ) has been found in familial cases of thrombocytosis. The change is serine to asparagine (S505N), which is an activating mutation. Screening for germline MPL mutations in pediatric cases of thrombocytosis identified S505N mutation in several families. It was also shown that there was a founder effect for the mutation. Although the patients have single lineage involvement and progenitors are not cytokine independent, the patients have some MPN-like properties, such as an increased risk of thrombotic events, splenomegaly, and progression of the disease to secondary myelofibrosis. Recently, another germline MPL mutation, Y252H, has been shown to be associated with thrombocytosis. Somatic MPL mutations (mostly W515) are found in some cases of ET and PMF.
Germline JAK2 mutations
Recently, several groups identified germline mutations in the JAK2 gene in familial cases of thrombocytosis. The mutations, V617I and R564Q, are located in the pseudokinase domain, in the vicinity of the V617F mutation. The authors have also identified a family with hereditary thrombocytosis, previously diagnosed as ET, to carry the germline JAK2-H608N mutation. All the families have a very similar phenotype, isolated thrombocytosis, absence of the somatic JAK2-V617F mutation, and high penetrance. This type of disease can also be considered MPN-like because it does not possess true MPN characteristics.
Other familial myeloid disorders
Malignant myeloid disorders other than MPN, such as myelodysplastic syndromes (MDS), juvenile myelomonocytic leukemia (JMML), and acute myeloid leukemia (AML), also show familial clustering. Several groups have studied those cases and identified germline mutations responsible for the development of the diseases. Inherited mutations in the CEBPA gene have been implicated in the development of AML, whereas germline mutations in GATA2 , RUNX1 , TERC, and TERT predispose to the development of MDS. These mutations explain a part of the familial cases of MDS and AML; although for the remaining proportion of families, predisposing mutations are yet to be identified.
It has been shown that germline mutations in CBL cause developmental abnormalities and predispose to JMML. Interestingly, CBL mutations are also acquired somatically in patients with JMML without familial history as well as in a wider spectrum of myeloid disorders, including MPN. Similarly, somatic RUNX1 mutations are detected in several patients with MDS and secondary AML. These facts illustrate the phenomenon of germline mutations in certain genes causing very specific neoplastic disorder, while the somatic mutations in the same gene are found in different related or even unrelated malignancies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree