Myeloproliferative neoplasms include 3 diseases: polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). PV and ET are dominated by a high risk of thrombosis and a late risk of clonal evolution into secondary MF and acute myeloid leukemia. Patients with PMF may encounter many complications associated with disease progression or with PMF evolution. This article defines factors that determine prognosis in these 3 diseases.
- •
Myeloproliferative neoplasms include 3 diseases: polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).
- •
PV and ET are dominated by a high risk of thrombosis and a late risk of clonal evolution into secondary MF and acute myeloid leukemia.
- •
Current risk prediction of PV and ET requires 2 parameters: age older than 60 years and prior history of thrombosis and, on the basis of these risk factors, patients are stratified as low risk or high risk.
- •
Patients with PMF may encounter many complications associated with disease progression or with PMF evolution.
- •
Concerning MF prognostication, the IPSS (International Prognostic Scoring System) model at diagnosis and the DIPSS (Dynamic IPSS) anytime during the course of the disease define survival of patients with MF; IPSS and DIPSS are based on age older than 65 years, presence of constitutional symptoms, hemoglobin level less than 10 g/dL, leukocyte count greater than 25 × 10 9 /L, and circulating blast cells 1% or greater.
Prognosis in ET
Survival of patients with ET is not significantly shortened when compared with that of the healthy population. Disease-related complications affecting survival are mainly vascular events (thrombosis and hemorrhage) and transformation to MF or AML. In ET, the incidence of thrombosis, MF, and AML was estimated at 12.0, 1.6, and 1.2 × 1000 person-years. Evolution to AML is difficult to predict, as it is a rare event, but advanced age (sign of genomic instability) and high leukocyte count (sign of myeloproliferation) at diagnosis are considered risk factors. Concerning the role of chemotherapy on leukemia occurrence in MPNs, a recent population-based study on 11,039 MPNs proved that 25% of patients post-MPN AML were never exposed to cytotoxic drugs, and that hydroxyurea at any dose is not associated with an increased risk of AML, whereas an increasing cumulative dose of alkylators is.
Prognostic Models in ET
As thrombosis is the most frequent complication in ET, there is a comprehensible rationale for stratifying patients according to the risk of thrombosis. There is a general agreement among investigators to consider age older than 60 years at diagnosis and presence of vascular events in the patient’s history as the 2 prognostic factors in ET as well as in PV ( Table 1 ). Therefore, patients with 1 or 2 of these risk factors at diagnosis are considered as high risk, whereas those with none of them are considered as low risk. This risk classification guides therapeutic strategy. Cardiovascular (CV) risk factors (arterial hypertension, smoking, hypercholesterolemia, diabetes) do not enter in the risk stratification of ET, but an appropriate strategy of prevention and management is recommended.
| Low Risk | Age <60 years and |
| No history of thrombosis | |
| High risk | Age ≥60 years, or |
| History of thrombosis |
Other Risk Factors in ET
Mutational profile
The mutational profile of patients with ET includes the JAK2 (V617F) mutation present in roughly 60% of patients, different mutations of MPL in 5%, and very few cases with TET2 mutations only. Many studies, recently reviewed, evaluated the relationship between the presence of the JAK2 (V617F) mutation and thrombosis occurrence during follow-up. Meta-analyses indicated that the risk of venous thrombosis and arterial thrombosis is 2.09-fold and 1.96-fold higher in JAK2 (V617F)-positive ET when compared with JAK2 (V617F)-negative ET. When studying allele burden, patients with homozygous mutations seem to have a higher risk of thrombosis (hazard ratio: 3.97) if compared with JAK2 -wt : it is notable that very few patients with ET (<2%) have a homozygous mutation of JAK2 . Mutations of MPL were reported in approximately 3% to 5% of patients with ET. MPL mutations do not define a distinct phenotype in ET, although patients with MPL (W515L/K) presented lower hemoglobin levels and higher platelet counts than did MPLwt. MPL mutation was demonstrated as a significant risk factor for microvessel disturbances, suggesting platelet hyperreactivity associated with constitutively active MPL. Studying bone marrow histopathology, patients with MPL (W515L/K) presented reduced total and erythroid bone marrow cellularity. Finally, MPL mutations lacked prognostic significance with respect to thrombosis, major hemorrhage, myelofibrotic transformation, or survival. TET2 mutations (frameshift, nonsense, or missense), mostly involving exons 4 and 12, have been found in 5% of patients with ET (mutant TET2 detected in 17%/7% of JAK2 [V617F]positive/negative MPN cases, respectively). The presence of mutant TET2 does not affect survival, leukemic transformation, or thrombosis in MPNs.
Cytogenetic abnormalities
Karyotypic abnormalities in ET are uncommon and occur in fewer than 5% of cases, with trisomy 9 and 8, del(13q) and del(20q), and unbalanced translocations as most common. A recent study of 402 patients with ET showed an association between abnormal cytogenetics at diagnosis and palpable splenomegaly, current tobacco use, venous thrombosis, and lower hemoglobin levels. Nevertheless, it was noted that these patients did not have a shorter survival, or an increased transformation to AML or MF. Very uncommon cytogenetic abnormalities, such as der(1;7)(q10;p10), define a subgroup of patients with ET with an unfavorable prognosis. Leukemic transformations are commonly associated with very poor prognostic abnormalities, including −5/5q− and −7/7q−.
Leukocytosis
A large retrospective cohort of 1063 patients with ET (193 patients [18%] with prior thrombosis), had 118 major thromboses (2.3% patients per year) during up to 38 years of follow-up. With regard to disease-related risk factors, the predictive role of baseline leukocyte levels was examined. Compared with patients having a leukocyte count lower than 8 × 10 9 /L, those with leukocyte count greater than 11 × 10 9 /L had a significantly higher risk of major thrombosis. This association seems particularly evident in younger and asymptomatic patients (low-risk category): ad hoc statistics indicated a higher risk of thrombosis in low-risk patients with leukocytosis, which figure was similar to that calculated in a conventionally defined high-risk group with a best leukocyte cutoff value of 9.4 × 10 9 /L. A study that dynamically evaluated the impact of leukocyte count variation over time in low-risk ET demonstrated that the increase of leukocytes implies a higher risk of subsequent thrombosis. Although some investigators found the same correlation, others did not disclose any relationship between leukocytosis and thrombosis.
Thrombocytosis
Extreme thrombocytosis per se might provoke an excess of bleeding because of acquired von Willebrand disease, and platelet counts of more than 1500 × 10 9 /L should be considered as a criterion to start cytoreduction in ET.
Bone marrow histopathology
After the first Polycythemia Vera Study Group (PVSG) criteria, the World Health Organization (WHO) dictated the new criteria for diagnosis of ET, which are essentially based on platelet count, histopathological features (normal age-matched bone marrow cellularity with dispersed large to giant megakaryocytes), and demonstration of clonality. The WHO histopathological criteria help to distinguish ET from another entity presenting with thrombocytosis: prefibrotic primary myelofibrosis (pPMF), characterized by increased age-matched bone marrow cellularity, dense to loose clustered atypical megakaryocytes, increased granulopoiesis, and reduced erythropoiesis. At first, the WHO histopathological criteria appeared not easy to apply, resulting in an insufficient distinction between ET and pPMF. More recently, an overall blinded consensus ranging from 88% (295 cases, 2 European centers) to 93% (1104 cases, 7 international centers) has been achieved among pathologists to recognize the 2 entities. The impact of a clear-cut identification of these 2 entities by histopathology is emphasized by clinical results of the international-based data collection of 1104 patients with a clinical phenotype of ET. Patients with WHO-defined ET showed a lower risk of overt myelofibrosis, AML evolution, and better survival when compared with patients with pPMF: the 10-year figures were 0.7%, 0.8%, and 89.0% for ET and 5.8%, 12.3%, and 76.0% for pPMF, respectively.
Model to Predict Survival in ET: International Prognostic System in ET
Very recently, a new prognostic score, International Prognostic System in ET (IPSET), has been developed. The prognostic impact on survival of age, prior thrombosis, leukocyte count, platelet count, hemoglobin level, JAK2 mutational status, and splenomegaly was tested in 867 patients with WHO-defined ET. Age older than 60 years, leukocyte count greater than 11 × 10 9 /L, and prior history of thrombosis were statistically significant on survival by multivariable analysis and were included in the new prognostic model. Each factor was assigned an integer weight close to the corresponding hazard ratio in the multivariable Cox regression: weight 2 for age older than 60 years; weight 1 for leukocyte count greater than 11 × 10 9 /L and for prior thrombosis. So, the IPSET model allocated patients into 3 risk categories with significantly different survival: low (sum of points = 0; median survival not reached), intermediate (sum = 1–2; median survival 24.5 years), and high (sum = 3–4, median survival 13.8 years).
Prognosis in PV
PV is a clonal myeloproliferative disorder characterized by the excessive production of red blood cells (RBCs) that in some cases is accompanied by leukocytosis and/or thrombocytosis. This deregulated proliferative pattern is sustained by the constitutive activation of the JAK-STAT signal transduction pathway through different mutations in the JAK2 gene: JAK2 (V617F) in approximately 95% of patients with PV and mutations within JAK2 exon 12 in approximately 4%. In a few cases, mutations of LNK or SOCS have been reported. The molecular characterization of PV has further been enriched by the identification of mutations in other potential epigenetic regulators, such as EZH2 and TET2, in approximately 3% of and in approximately 16% of JAK2 (V617F)-positive patients with PV, respectively. Except for JAK2 exon 12 mutations, none of the above-cited molecular alterations is PV-specific.
Prognostic Models in PV
Patients with PV have a shortened life expectancy when compared with that of the general population. The most frequent complication is thrombosis (with arterial thrombotic events being predominant), with an incidence estimated at 18 × 1000 person-years and accounting for 45% of all deaths; whereas evolution to post-PV MF and to AML both occur with an incidence of 5 × 1000 person-years and account for 13% of all deaths. Considering the type and frequency of disease-related complications, and the absence to date of effective long-term treatments able to significantly reduce or eliminate JAK2 (V617F), patients tend to be stratified per thrombotic risk and treated accordingly. Among others, the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) showed that age and history of thrombosis were the main predictors of CV events. In detail, this study reported that patients younger than 65 years without prior thrombosis have an incidence of thrombosis of 2.5 × 100 persons per year, those older than 65 years or with prior thrombosis have an incidence of 5.0 × 100 persons per year, and patients older than 65 years with prior thrombosis have an incidence of 10.9 × 100 persons per year. The current risk stratification system includes age older than 60 years at diagnosis and previous history of vascular events: patients with at least one of these risk factors at diagnosis are considered high risk, as opposed to those with no risk factors, who are classified as low risk. This risk stratification applies to all patients with PV, regardless of the type of JAK2 mutation they harbor.
Classic CV risk factors, such as arterial hypertension, smoking, hypercholesterolemia, diabetes, and obesity, although not included in the risk-stratification model, can enhance CV risk and should be minimized (smoking has been demonstrated to increase thrombotic risk in PV).
Other Prognostic Risk Factors in PV
Hematocrit value and platelet count
The optimal hematocrit target to pursue in PV will be clarified by the ongoing CYTOreductive Therapy to Prevent Cardiovascular Events in Patients with Polycythemia Vera trial : for the time being the European LeukemiaNet guidelines suggest to lower the hematocrit below 45%, even though the 2 largest studies in PV, namely the ECLAP and the Polycythemia Vera Study Group-01 studies, did not identify a benefit in maintaining hematocrit values below 50% to 52%.
Thrombocytosis has not been demonstrated to increase thrombotic risk and, when platelets exceed 1500 × 10 9 /L, may even confer an increased bleeding risk, owing to acquired von Willebrand disease, and therefore warrants caution.
Leukocytosis
Many investigators have studied the relationship between leukocytosis and thrombosis. A positive correlation between these 2 factors has been found in some, but not in all, studies. The ECLAP study found a significant increase of myocardial infarction in patients with leukocyte counts exceeding 15 × 10 9 /L versus those having leukocyte counts below 10 × 10 9 /L. In addition, a leukocyte count exceeding 15 × 10 9 /L has been found associated with a higher risk of evolution into post-PV MF.
Mutational status
Regarding JAK2 (V617F) allele burden, one study reported that patients harboring greater than 75% JAK2 (V617F) allele had significantly increased relative risk of total thrombosis (at diagnosis and in the follow-up) and of thrombosis occurring during follow-up, whereas the difference did not reach the significance levels for thrombosis occurring at diagnosis. Other retrospective and prospective analysis revealed no correlation between thrombosis and increasing allele burden.
Concerning TET2 mutations, reported in 16% of all cases of PV, the presence of mutant TET2 does not affect survival, leukemic transformation, or thrombosis.
A recent and very interesting study has shown that individual genetic variability may play a role in AML transformation. A polymorphism in the XPD gene (involved in the nucleotide excision repair pathway), namely Lys751Gln, has been found to be an independent risk factor for leukemic transformation in ET and PV. In detail, homozygous carriers of the minor allele (Gln/Gln) of this polymorphism have a nearly fivefold higher risk of progression to AML compared with patients harboring the other XPD genotypes.
Bone marrow fibrosis
Prevalence and prognostic relevance of bone marrow reticulin fibrosis was assessed in 526 patients with WHO-defined PV. At diagnosis, 14% of the patients displayed grade 1 reticulin fibrosis. Patients with fibrosis were less prone to experience thrombosis during their clinical course (1.1 vs 2.7 per 100 patient-years) and more prone to develop post-PV MF (2.2 vs 0.8 per 100 patient-years). There was no significant difference between the 2 groups in terms of overall or leukemia-free survival.
Model to Predict Survival in PV
A model to predict survival has been reported in PV, indicating that advanced age, leukocytosis, and prior venous thrombosis affect survival. As an example, based on this model, a 60-year-old patient with PV with leukocytosis or with prior venous thrombosis has an expected median survival of approximately 11 years.
Prognosis in PV
PV is a clonal myeloproliferative disorder characterized by the excessive production of red blood cells (RBCs) that in some cases is accompanied by leukocytosis and/or thrombocytosis. This deregulated proliferative pattern is sustained by the constitutive activation of the JAK-STAT signal transduction pathway through different mutations in the JAK2 gene: JAK2 (V617F) in approximately 95% of patients with PV and mutations within JAK2 exon 12 in approximately 4%. In a few cases, mutations of LNK or SOCS have been reported. The molecular characterization of PV has further been enriched by the identification of mutations in other potential epigenetic regulators, such as EZH2 and TET2, in approximately 3% of and in approximately 16% of JAK2 (V617F)-positive patients with PV, respectively. Except for JAK2 exon 12 mutations, none of the above-cited molecular alterations is PV-specific.
Prognostic Models in PV
Patients with PV have a shortened life expectancy when compared with that of the general population. The most frequent complication is thrombosis (with arterial thrombotic events being predominant), with an incidence estimated at 18 × 1000 person-years and accounting for 45% of all deaths; whereas evolution to post-PV MF and to AML both occur with an incidence of 5 × 1000 person-years and account for 13% of all deaths. Considering the type and frequency of disease-related complications, and the absence to date of effective long-term treatments able to significantly reduce or eliminate JAK2 (V617F), patients tend to be stratified per thrombotic risk and treated accordingly. Among others, the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) showed that age and history of thrombosis were the main predictors of CV events. In detail, this study reported that patients younger than 65 years without prior thrombosis have an incidence of thrombosis of 2.5 × 100 persons per year, those older than 65 years or with prior thrombosis have an incidence of 5.0 × 100 persons per year, and patients older than 65 years with prior thrombosis have an incidence of 10.9 × 100 persons per year. The current risk stratification system includes age older than 60 years at diagnosis and previous history of vascular events: patients with at least one of these risk factors at diagnosis are considered high risk, as opposed to those with no risk factors, who are classified as low risk. This risk stratification applies to all patients with PV, regardless of the type of JAK2 mutation they harbor.
Classic CV risk factors, such as arterial hypertension, smoking, hypercholesterolemia, diabetes, and obesity, although not included in the risk-stratification model, can enhance CV risk and should be minimized (smoking has been demonstrated to increase thrombotic risk in PV).
Other Prognostic Risk Factors in PV
Hematocrit value and platelet count
The optimal hematocrit target to pursue in PV will be clarified by the ongoing CYTOreductive Therapy to Prevent Cardiovascular Events in Patients with Polycythemia Vera trial : for the time being the European LeukemiaNet guidelines suggest to lower the hematocrit below 45%, even though the 2 largest studies in PV, namely the ECLAP and the Polycythemia Vera Study Group-01 studies, did not identify a benefit in maintaining hematocrit values below 50% to 52%.
Thrombocytosis has not been demonstrated to increase thrombotic risk and, when platelets exceed 1500 × 10 9 /L, may even confer an increased bleeding risk, owing to acquired von Willebrand disease, and therefore warrants caution.
Leukocytosis
Many investigators have studied the relationship between leukocytosis and thrombosis. A positive correlation between these 2 factors has been found in some, but not in all, studies. The ECLAP study found a significant increase of myocardial infarction in patients with leukocyte counts exceeding 15 × 10 9 /L versus those having leukocyte counts below 10 × 10 9 /L. In addition, a leukocyte count exceeding 15 × 10 9 /L has been found associated with a higher risk of evolution into post-PV MF.
Mutational status
Regarding JAK2 (V617F) allele burden, one study reported that patients harboring greater than 75% JAK2 (V617F) allele had significantly increased relative risk of total thrombosis (at diagnosis and in the follow-up) and of thrombosis occurring during follow-up, whereas the difference did not reach the significance levels for thrombosis occurring at diagnosis. Other retrospective and prospective analysis revealed no correlation between thrombosis and increasing allele burden.
Concerning TET2 mutations, reported in 16% of all cases of PV, the presence of mutant TET2 does not affect survival, leukemic transformation, or thrombosis.
A recent and very interesting study has shown that individual genetic variability may play a role in AML transformation. A polymorphism in the XPD gene (involved in the nucleotide excision repair pathway), namely Lys751Gln, has been found to be an independent risk factor for leukemic transformation in ET and PV. In detail, homozygous carriers of the minor allele (Gln/Gln) of this polymorphism have a nearly fivefold higher risk of progression to AML compared with patients harboring the other XPD genotypes.
Bone marrow fibrosis
Prevalence and prognostic relevance of bone marrow reticulin fibrosis was assessed in 526 patients with WHO-defined PV. At diagnosis, 14% of the patients displayed grade 1 reticulin fibrosis. Patients with fibrosis were less prone to experience thrombosis during their clinical course (1.1 vs 2.7 per 100 patient-years) and more prone to develop post-PV MF (2.2 vs 0.8 per 100 patient-years). There was no significant difference between the 2 groups in terms of overall or leukemia-free survival.
Model to Predict Survival in PV
A model to predict survival has been reported in PV, indicating that advanced age, leukocytosis, and prior venous thrombosis affect survival. As an example, based on this model, a 60-year-old patient with PV with leukocytosis or with prior venous thrombosis has an expected median survival of approximately 11 years.
Prognosis in PMF
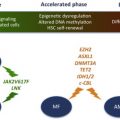
Among MPNs, PMF has the most heterogeneous clinical presentation, which may encompass anemia, splenomegaly, leukocytosis or leukopenia, thrombocytosis or thrombocytopenia, and constitutional symptoms. Median survival in PMF is estimated at 6 years, but it can range from a few months to many years. Causes of death may be recapitulated into bone marrow failure (severe anemia, bleeding caused by thrombocytopenia, and infections because of leukopenia) in 25% to 30% of patients, leukemic transformation, named blast phase (BP), in 10% to 20% of patients, CV complications in 15% to 20%, and portal hypertension in 10%. Increasing rates of secondary malignancy have been reported in patients with a longer follow-up.
Many factors affect survival in PMF, such as advanced age, anemia, RBC transfusion need, leukopenia, leukocytosis, thrombocytopenia, peripheral blast count, systemic symptoms, hepatic myeloid metaplasia, decreased marrow cellularity with higher degree of fibrosis, higher degree of microvessel density, high number of circulating CD34-positive cells, cytogenetic abnormalities, the JAK2 (V617F) mutation, EZH2 mutation, and high level of some cytokines.
Prognostic Models at Diagnosis of PMF
In the past years, many prognostic models have been developed in PMF, but the most used was the Lille score, recently replaced with the International Prognostic scoring System (IPSS). The progressive nature of PMF generated interest in defining new so-called dynamic models, such as the dynamic-IPSS (DIPSS) and the most recent DIPSS-Plus.
The Lille score
The Lille score model was designed on 195 patients with PMF, who had a median survival of 42 months. The scoring system was based on hemoglobin less than 10 g/dL and leukocyte count lower than 4 × 10 9 /L or greater than 30 × 10 9 /L. Patients were grouped into 3 categories, low (0 factor), intermediate (1 factor), and high (2 factors) risk, associated with a median survival of 93, 26, and 13 months, respectively. The study also revealed that an abnormal karyotype (present in ∼35% of patients) was associated with a shortened survival , especially in the low-risk group. Leukocyte count greater than 30 × 10 9 /L and abnormal karyotype predict evolution to BP.
The IPSS score
The IPSS was defined through the collaboration of 7 centers under the auspices of the International Working Group on MPN Research and Treatment (IWG-MRT) in 2009. After a systematic individual case review, the database included 1054 patients with PMF defined according to the WHO classification system, excluding post-PV and post-ET MF and pPMF. This is the largest prognostic study ever performed in PMF. Median survival was 69 months. Multivariate analysis of parameters obtained at disease diagnosis identified age older than 65 years, presence of constitutional symptoms, hemoglobin level less than 10 g/dL, leukocyte count greater than 25 × 10 9 /L, and circulating blast cells 1% or greater as predictors of shortened survival. Based on the presence of 0 (low risk), 1 (intermediate risk-1), 2 (intermediate risk-2), or greater than or equal to 3 (high risk) of these variables, 4 risk groups with no overlapping in their survival curves were generated ( Table 2 ). The 4 risk categories were well balanced: 22% in low risk, 29% in intermediate risk-1, 28% in intermediate risk-2, and 21% in high risk. Median survivals were 135 months for low-risk patients, 95 months for intermediate-1 patients, 48 months for intermediate-2 patients, and 27 months for high-risk patients.