Robotic-assisted pulmonary lobectomy can be considered for patients able to tolerate conventional lobectomy. Contraindications to resection via thoracotomy apply to patients undergoing robotic lobectomy. Team training, familiarity with equipment, troubleshooting, and preparation are critical for successful robotic lobectomy. Robotic lobectomy is associated with decreased rates of blood loss, blood transfusion, air leak, chest tube duration, length of stay, and mortality compared with thoracotomy. Robotic lobectomy offers many of the same benefits in perioperative morbidity and mortality, and additional advantages in optics, dexterity, and surgeon ergonomics as video-assisted thoracic lobectomy. Long-term oncologic efficacy and cost implications remain areas of study.
Key points
- •
Robotic pulmonary lobectomy is becoming an increasingly common modality used for lung resection, and can be done with very low morbidity and mortality.
- •
Robotic lobectomy offers similar benefits to video-assisted thoracic surgery (VATS) lobectomy in postoperative recovery, and additional advantages in optics, dexterity, and surgeon ergonomics.
- •
Further comparisons between VATS and robotics in terms of ease of training, long-term oncologic efficacy, and patient outcomes are an area of active study.
Introduction
Video-assisted thoracic surgery (VATS) for pulmonary resection, including lobectomy, has become widely used among general thoracic surgeons. However, robotic surgery has certain advantages, such as 3-dimensional visualization, improved camera quality, wristed instruments, and ergonomic ease. As such, the performance of robotic-assisted pulmonary lobectomy has increased since it was first reported in the English literature in 2003. Robotic surgery has certain limitations, including more complex setup time, increased costs, absence of haptic/tactile feedback, and the need for specialized equipment and training. However, results of completely portal robotic lobectomy as done with the 4-arm technique (CPRL-4) similar to or as described by Cerfolio and colleagues have been shown to yield excellent results in terms of both intraoperative criteria and postoperative morbidity and mortality.
Introduction
Video-assisted thoracic surgery (VATS) for pulmonary resection, including lobectomy, has become widely used among general thoracic surgeons. However, robotic surgery has certain advantages, such as 3-dimensional visualization, improved camera quality, wristed instruments, and ergonomic ease. As such, the performance of robotic-assisted pulmonary lobectomy has increased since it was first reported in the English literature in 2003. Robotic surgery has certain limitations, including more complex setup time, increased costs, absence of haptic/tactile feedback, and the need for specialized equipment and training. However, results of completely portal robotic lobectomy as done with the 4-arm technique (CPRL-4) similar to or as described by Cerfolio and colleagues have been shown to yield excellent results in terms of both intraoperative criteria and postoperative morbidity and mortality.
Indications
Robotic-assisted pulmonary lobectomy can be considered for any patient that is deemed fit to tolerate conventional lobectomy. In fact, patients have a better functional outcome after minimally invasive compared with open lobectomy; therefore, patients considered marginal or unfit for thoracotomy may still be considered for robotic lobectomy. Burt and colleagues have shown that VATS lobectomy can be performed with predicted postoperative forced expiratory volume in 1 second or diffusion capacity of the lung for carbon monoxide of less than 40% with acceptable morbidity and mortality. We believe that robotic-assisted lobectomy is similarly safe in this group of patients that had traditionally been considered inoperable.
Tumors larger than 7 cm (T3), tumors crossing fissures, and centrally located tumors may all be considered for robotic lobectomy with proper patient selection and increasing surgeon experience, but in general these factors disfavor a robotic approach. Similarly, radiologic evidence of N1 nodes, induction chemotherapy and/or radiation, calcified lymph nodes and prior thoracic surgery are not contraindications to robotic lobectomy but a robotic approach to these patients should not be selected early in one’s learning curve.
Chest wall resection with robotic assistance for the parenchymal resection part of the procedure is feasible as well. VATS chest wall resection has been shown to be safe and to decrease the extent of reconstruction necessary. As of yet, however, there are no published series of robotic-assisted combined lung–chest wall resections. The postoperative benefits of minimally invasive chest wall resection remain unclear.
Completely robotic sleeve lobectomy and resection with bronchoplasty have been performed at our institution, but only a single hybrid robotic–VATS case has been published from another center. It is possible that the results from these operations will prove favorable; however, at this time we recommend that only practitioners with extensive experience with robotic thoracic surgery attempt these procedures. Complex vascular reconstruction remains preferentially approached from a thoracotomy.
The typical contraindications for lobectomy that apply to patients undergoing resection via thoracotomy would also apply to patients undergoing robotic lobectomy (eg, prohibitive lung function or medical comorbidities, multistation N2, gross N2 disease, or evidence of N3 disease). Patients with Pancoast tumors, tumors with extensive invasion into the mediastinum or esophagus, and those with contraindications to general anesthesia or single-lung ventilation are also poor candidates for robotic lobectomy. In addition, small nodules that are not tissue diagnosed that require lung palpation for wedge resection are considered by some as a contraindication for robotic lobectomy when a completely portal technique is used because of the inability to palpate the lung; however, lung palpation is possible with a robotic technique when a robotic-assisted technique is used. However, we have used navigational bronchoscopy with methyl blue tattooing of the nodules to help guide wedge resection or a robotic lymph node dissection and then conversion to VATS.
Equipment
The Da Vinci Surgical System is currently the only robotic system approved by the US Food and Drug Administration for lung surgery. The surgeon sits at a console some distance from the patient, who is positioned on an operating table in close proximity to the robotic unit with its 4 robotic arms. The robotic arms incorporate remote center technology, in which a fixed point in space is defined, and about it the surgical arms move so as to minimize stress on the thoracic wall during manipulations. The small proprietary Endowrist instruments attached to the arms are capable of a wide range of high-precision movements. These are controlled by the surgeon’s hand movements, via “master” instruments at the console. The master instruments sense the surgeon’s hand movements and translate them electronically into scaled-down micromovements to manipulate the small surgical instruments. Hand tremor is filtered out by a 6-Hz motion filter. The surgeon observes the operating field through console binoculars. The image comes from a maneuverable high-definition stereoscopic camera (endoscope) attached to one of the robot arms. The console also has foot pedals that allow the surgeon to engage and disengage different instrument arms, reposition the console master controls without the instruments themselves moving, and activate electric cautery. A second optional console allows tandem surgery and training.
Preoperative evaluation
Preoperative evaluation including pulmonary function testing should be obtained. Patients who are borderline in terms of overall health and/or respiratory parameters can undergo exercise testing to determine maximal oxygen consumption (V o 2 max); patients with a V o 2 max of greater than 15 mL/kg*min are considered moderate risk, 10 to 15 mL/kg*min are high risk, and less than 10 mL/kg*min are prohibitive risk. We routinely obtain stress testing to assess for myocardial ischemia especially in patients who have had a significant smoking history. Complete patient-specific staging should also be performed before lung resection. This includes PET-computed tomography scanning in almost all patients and the selective use of brain MRI or computed tomography (those who are symptomatic or who have large central adenocarcinomas), endobronchial ultrasound-guided fine needle aspiration, esophageal ultrasound-guided fine needle aspiration for biopsy of the posterior inferior lymph nodes and adrenals, and/or mediastinoscopy depending on the tumor size, location, radiologic findings, and institutional experience.
Port placement
Robotic lobectomy is a technique that can be applied to a broad range of patients and tumors. Although small, peripheral tumors in thin patients are the most easily dealt with robotically, obesity, large size of tumor, and central location of tumor are not contraindications for robotic resection.
There are several different techniques used to perform the operation. Veronesi and Melfi in 2010 reported the safety of a 4-arm robotic assisted (not completely portal) lobectomy (using a 3- to 4-cm access incision as used by VATS surgeons) in 54 patients. Ninan and Dylewski in 2010 reported the effectiveness of a completely portal robotic lobectomy using 3 arms (CPRL-3) in 74 patients. Gharagozloo and colleagues in 2009 reported his outcomes using a hybrid technique.
We prefer the CPRL-4 method. The technique with an SI robot is performed in the following manner for a right upper lobe. For a lower lobe the exactly same sequence is used, but the ports are placed over the ninth ribs not the eighth.
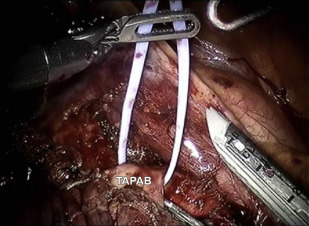
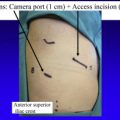
The port that is placed first is the camera port. We prefer to use a 5-mm VATS camera as opposed to the 8- or 12-mm robotic camera in case the underlying lung is injured. Once the pleural space is entered over the top of the eighth rib, warm humidified CO 2 is insufflated into the chest and a 5-mm camera is placed via the camera port and an extensive subpleural paravertebral block is carried out using 0.25% Marcaine with epinephrine from ribs 3 to 12. The next port that is, placed is the 5-mm most posterior port, which is always used for robotic arm 3; this is located 2 to 3 cm lateral to the spinous process of the vertebral bodies. As shown in Fig. 1 , which is a right-sided resection, robotic arm 3 represents the second left hand. If you were operating in the left chest robotic, arm 3 would represent a second right-handed instrument.
The next port that is placed is robotic arm number 2, which always serves as the left hand when using an SI robot. We now currently use a 5-mm robotic DeBakey instrument or a Shertell for our left hand and used to use an 8-mm port and use a Caudierre. Once this trocar is placed only 2 trocars are left. They are carefully planned before making any incisions in the skin. The goal of these 2 trocars is to maximize the distance between the access port and the port for robotic arm 1. This affords the greatest working area for the assistant. Using a needle and injecting Marcaine into the chest, these sites are chosen by placing the access port (a 12-mm plastic disposable trocar) as low in the chest as possible and just above the diaphragmatic fibers so as not to injure it. This incision is not made until the site for robotic arm 1 is chosen as well. Note that these ports (the camera, the access port, and the site for robotic arm 1) form an isosceles triangle (see Fig. 1 ).
We use only a 0° camera to avoid injuring the intercostal nerve. Through the access port we place a 12-mm plastic port and through robotic arm 1 we use an 8-mm metal trocar and place a bipolar instrument.
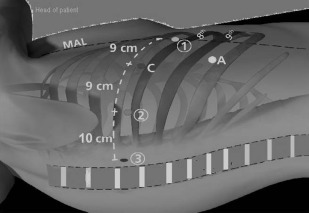
For the Xi model, there are few changes in the setup. The reusable trocars come currently in 2 sizes; 8 mm is the standard size and a 12 mm, which is used for the robotic stapler. It affords easier docking technology, but one cannot use other trocar models for the robotic arm ( Fig. 2 ). We begin by placing a robotic 8-mm trocar in the camera site, which could be above the eighth or ninth rib. We find that entering the chest cavity a rib space lower on the Xi affords improved visualization and also the arms can reach further on the Xi than Si so there are no downsides in placing the trocars lower when performing upper lobes. For the posterior port, an 8-mm trocar is placed and a double fenestrated tip up instrument is used in lieu of the thoracic 5-mm grasper, which is not available yet for the Xi. Finally, a 12-mm trocar is used for arm number 2 for right-sided resections or arm 3 for left-sided resections, and is used for the robotic vascular as well as thick tissue loads. There is an inner cannula that downsizes the 12-mm trocar to an 8-mm one for use of the other robotic instruments or camera “hopping” if needed. Alternately, 8-mm trocars can be used for all the robotic and camera arms if the robotic stapler is not used. The robot can come from the patient’s sides and docked because it has a rotating boom positioned, which affords the anesthesia staff improved access to the airway ( Fig. 3 ).
Technique
After the pleural surface is inspected to confirm the absence of metastases, we proceed with mediastinal lymph node dissection.
Right Side
The inferior pulmonary ligament is divided to get to lymph node station 9. It is removed along with lymph node station 8. Robotic arm 3 is used to retract the lower lobe medially and anteriorly to remove lymph nodes from station 7. Care is taken to control the 2 feeding arteries that make the subcarinal lymph node bloody. Robotic arm 3 is used to retract the upper lobe inferiorly and robotic arms 1 and 2 are used to dissect out stations 2R and 4R, clearing the space between the superior vena cava anteriorly, the esophagus posteriorly, and the azygos vein inferiorly. Avoiding dissection too far superiorly can prevent injury to the right recurrent laryngeal nerve that wraps around the subclavian artery.
Left Side
The inferior pulmonary ligament is divided to facilitate the removal of lymph node station 9. The nodes in station 8 are then removed. Station 7 is accessed in the space between the inferior pulmonary vein and lower lobe bronchus, lateral to the esophagus. If still in position, the lower lobe is retracted medially/anteriorly with robotic arm 3 during this process. Absence of the lower lobe facilitates dissection of level 7 from the left. Finally, robotic arm 3 is used to wrap around the left upper lobe and press it inferiorly to allow dissection of stations 5 and 6. Care should be taken while working in the aortopulmonary window to avoid injury to the left recurrent laryngeal nerve. Station 2L cannot typically be accessed during left sided mediastinal lymph node dissection owing to the presence of the aortic arch, but the 4L node is commonly removed.
Once the absence of mediastinal lymph node metastases is confirmed, we then proceed with pulmonary lobectomy. In situations where a confirmatory preoperative biopsy has not been done and the tumor is peripheral, a wedge resection may be done to establish a definitive diagnosis of a cancer before going ahead with lobectomy.
The order of structures to be isolated and divided is not always the same; however, certain patterns emerge depending on the lobe to be removed. A few principles, however, are worth keeping in mind:
- •
Lymph nodes in interlobar and intersegmental lesions are removed first to facilitate dissection of the vascular structures and bronchi.
- •
Bipolar cautery may be used to dissect the fissure with minimal air leak. As opposed to VATS, which generally emphasizes stapling of the fissure as one of the last steps of lobectomy, for robotic lobectomies the fissure is often approached first so that vascular structures can be identified and isolated.
- •
It is helpful to encircle the structures to be divided with vessel loops before attempting to pass the stapler. The stapler should be passed through the port that offers the best angle of attack, whether it is robot arm #1, #2, or the assistant port. If using a robot port, the port itself will have to be removed before putting the stapler through.
Wedge resection
Wedge resection of a nodule may be necessary to confirm the presence of cancer before proceeding with lobectomy. Because the current iteration of the robot does not offer tactile feedback, special techniques may be necessary to identify a nodule that is not obvious on visual inspection. An empty ring forceps may be used via the assistant port to palpate the nodule. Alternatively, preoperative marking of the nodule with a dye marker injected via navigational bronchoscopy can facilitate location of the nodule. Preoperative confirmation of a cancer diagnosis with tissue biopsy is helpful to avoid being unable to locate the nodule intraoperatively.
The 5 lobectomies
A certain degree of adaptability is necessary for performance of robotic lobectomy. Structures may be isolated and divided in the order that the patient’s individual anatomy permits. What follows is a description of an outline of the typical conduct of each lobectomy.
Right Upper Lobectomy
- •
Retraction of the right upper lobe laterally and posteriorly with robot arm 3 helps to expose the hilum.
- •
The bifurcation between the right upper and middle lobar veins is developed by dissecting it off the underlying pulmonary artery.
- •
The 10R lymph node between the truncus branch and the superior pulmonary vein should be removed or swept up toward the lung, which exposes the truncus branch.
- •
The superior pulmonary vein is encircled with the vessel loop and then divided ( Figs. 4 and 5 ). The truncus branch is then divided.