Chapter Outline
Cellular Origins of Retinoblastoma
Retinoblastoma Tumor Cell Differentiation
Retinoblastoma Genomics and Epigenomics
Retinoblastoma Preclinical Models
GENETIC COUNSELING FOR FAMILIES WITH RETINOBLASTOMA
CLINICAL MANIFESTATIONS OF RETINOBLASTOMA
DIAGNOSIS AND EXTENT OF DISEASE EVALUATIONS IN RETINOBLASTOMA
PRINCIPLES OF TREATMENT OF RETINOBLASTOMA
TREATMENT OF INTRAOCULAR RETINOBLASTOMA
TREATMENT OF EXTRAOCULAR RETINOBLASTOMA
LONG-TERM EFFECTS OF RETINOBLASTOMA AND ITS TREATMENT
Orbital Growth and Facial Asymmetry
Cognitive and Functional Development of Patients with Retinoblastoma
Retinoblastoma is the most frequent neoplasm of the eye in childhood and the third most common intraocular malignancy in all ages, following malignant melanoma and metastatic carcinoma. Retinoblastoma represents 2.5% to 4% of all pediatric cancers but 11% of cancers in the first year of life. The average age-adjusted incidence rate of retinoblastoma in the United States and Europe is 2 to 5 per million children (approximately one in 14,000 to 18,000 live births). Thus retinoblastoma develops in an estimated 8000 children each year worldwide.
Retinoblastoma is a cancer of the very young; two thirds of all cases of retinoblastoma are diagnosed before 2 years of age, and 95% of cases are diagnosed before 5 years of age. For these reasons, in addition to cure of the disease, therapeutic approaches require consideration of the need to preserve vision with minimal long-term adverse effects.
Retinoblastoma presents in two distinct clinical forms. The first form is bilateral or multifocal and heritable (occurring in 25% of all cases of retinoblastoma); it is characterized by the presence of germline mutations of the retinoblastoma-1 (RB1) gene. Multifocal retinoblastoma may be inherited from an affected survivor (25%), or it may be the result of a new germline mutation (75%). The second form is unilateral (representing 75% of all cases); about 85% to 90% of the cases are nonhereditary, and the remainder carry a germline RB1 mutation.
Epidemiology
The incidence of retinoblastoma is not distributed equally around the world. It appears to be higher (6 to 10 cases per million children) in Africa and India and among children of Native American descent in the North American continent. The increased incidence in those groups occurs primarily at the expense of unilateral cases. Whether these geographic variations are due to ethnic or socioeconomic factors is not known. However the fact that even in industrialized countries an increased incidence of retinoblastoma is associated with poverty and low levels of maternal education suggests a role for the environment. Differences in the incidence of embryonal tumors between countries and ethnic groups have been consistently reported during the past decades, particularly for retinoblastoma ; however the deficiencies inherent to suboptimal cancer registries in low-income countries have made proper estimates difficult. In a country as ethnically diverse and socioeconomically heterogenous as Brazil, De Camargo and colleagues calculated the age-adjusted incidence rates (AAIR) of retinoblastoma in 20 population-based cancer registries and noted higher AAIRs than those of developed countries. The AAIR for children 0 to 4 years of age was as high as 15 and 27 in Salvador and Bahia, respectively, two of the most deprived states in the country (versus 10 to 12 in the United States and Europe). In a broader study testing the differences in incidence for embryonal tumors and their correlation with socioeconomic status, the same group documented an inverse correlation between the incidence of retinoblastoma and socioeconomic index. A similar phenomenon has been reported in Mexico, where a higher incidence of retinoblastoma has been documented, particularly in deprived states such as Chiapas.
Decreased dietary intake of vegetables and fruits during pregnancy, resulting in decreased intake of nutrients such as folate and carotenoids, which are necessary for deoxyribonucleic acid (DNA) methylation and synthesis and for retinal formation, has also been associated with an increased risk of unilateral sporadic retinoblastoma. In a case-control study, the risk of developing retinoblastoma was associated with a maternal polymorphism in dihydrofolate reductase ( DHFR 19bpdel), particularly in women taking prenatal synthetic folic acid supplements.
It is well known that exposure to certain toxic agents during gestation increases the frequency of germinal mutations in animals. The vast majority of germline mutations in sporadic heritable retinoblastoma are paternally derived, and studies have suggested an association between paternal age and occupation with the occurrence of sporadic heritable retinoblastoma. Reports have also suggested an association between retinoblastoma and increased sunlight exposure, air toxins from gasoline and diesel combustion, or in vitro fertilization. In a case-control study of sporadic retinoblastoma, radiologic studies of the abdomen leading to scattered radiation exposure of the gonads was associated with an increased risk of bilateral retinoblastoma in the child.
Retinoblastoma tumors arise from fetal retinal cells that have lost function of both allelic copies of the RB1 gene, the first of the tumor suppressor genes to be cloned. The first event may be either a germline or a somatic mutation, but the second and subsequent events are always somatic. Not all tumors have mutations in RB1 , suggesting that there is either another gene or alternate mechanisms for inactivation of RB1 function. For example, the RB1 gene can be epigenetically silenced through hypermethylation of the promoter. In recent years, studies have suggested a role for human papillomaviruses (HPVs) in the pathogenesis of retinoblastoma. The viral oncoprotein E7 of high-risk HPV types has been shown to bind to and inactivate the RB1 gene product. Therefore it is plausible that HPV infection could be functionally equivalent to the biallelic loss of RB1 . Transgenic mice expressing HPV16 E6 and E7 proteins develop retinoblastoma. Presumably exposure to HPV would occur peripartum from genital infection of the mother. In this regard the use of barrier methods of contraception is associated with a reduced incidence of both retinoblastoma and HPV infection, and studies have also shown a correlation with sexually transmitted diseases during pregnancy. Interestingly an overlap occurs between countries in which the relative incidence of retinoblastoma is greatest and those in which the incidence of cervical carcinoma is highest. High-risk HPV sequences have been detected in 28% to 36% of tumors in some populations.
Biology of Retinoblastoma
Cellular Origins of Retinoblastoma
It is widely believed that the molecular and cellular features of a tumor reflect its cell of origin and can thus provide clues about treatment targets. Since 1897 when Wintersteiner first proposed that rosettes in retinoblastoma are reminiscent of photoreceptor differentiation, the cell of origin and the cellular features of retinoblastoma have been debated. Initially the focus was on the cellular features that could be identified by histologic analyses, such as the presence of rosettes. With advances in molecular biology and biochemistry, the debate about the retinoblastoma cell of origin has been revisited, with a strong predilection toward photoreceptors being the cell of origin. In addition to photoreceptors, a number of independent studies suggested that retinoblastomas may also have features of progenitor cells or interneurons such as amacrine cells. The possibility of tumor cells with a hybrid molecular/cellular phenotype was not considered in those previous studies.
In a recent study of the molecular, cellular, neuroanatomic, and neurochemical features of mouse and human retinoblastoma, it was shown that they have features of multiple cell classes, principally amacrine/horizontal interneurons, retinal progenitor cells, and photoreceptors. Indeed, single-cell gene expression array analysis showed that these multiple cell type-specific developmental programs are coexpressed in individual retinoblastoma cells, which creates a progenitor/neuronal hybrid cell. It is widely believed that the initiating genetic lesion in the RB1 gene occurs in proliferating retinal progenitor cells during fetal development. Retinal progenitor cells may be the cell of origin for retinoblastoma, and the multiple differentiation programs that are found in human and mouse retinoblastomas may reflect the multipotent competence of retinal progenitor cells. Indeed in single-cell gene expression array analyses of mouse retinal progenitor cells, photoreceptor and interneuron genes were often expressed in proliferating retinal progenitor cells.
The finding that retinoblastoma tumor cells express multiple neuronal differentiation programs that are normally incompatible in development suggests that the pathways that control retinal development and establish distinct cell types are perturbed during tumorigenesis. This discovery underscores the challenges of assigning cell-of-origin status without first establishing the full molecular and cellular signature of a tumor. It is important to emphasize that these studies do not necessarily indicate that a photoreceptor, interneuron, or progenitor cell is the cell of origin for retinoblastoma. Detailed cell-lineage studies combined with live imaging approaches will be required to definitively identify the cell of origin for retinoblastoma. Nonetheless, this detailed characterization of the neuronal pathways that are deregulated in retinoblastoma may provide novel targets for therapeutic intervention. This example highlights the importance of comprehensive molecular, cellular, and physiologic characterization of human cancers with single-cell resolution as we incorporate molecular targeted therapy into treatment regimens.
Retinoblastoma Tumor Cell Differentiation
Many of the previous studies on retinoblastoma tumor cell differentiation have focused on photoreceptor features, including a recent study linking the origin of retinoblastoma to cone photoreceptors. As previously described, a detailed cross-species gene expression array analysis indicated that retinoblastomas express molecular features of photoreceptors and that the rod signature is more robust than the cone signature. However morphometric and neuroanatomic studies of mouse and human retinoblastomas showed few cellular features of photoreceptors, which raises the possibility that the gene regulatory network that controls photoreceptors is deregulated in human retinoblastomas. Interestingly a large number of photoreceptor genes are also expressed in medulloblastomas despite their distinct cellular origins, which may indicate that the transcriptional pathways involved in photoreceptor differentiation are nonspecifically deregulated in a variety of tumors of the central nervous system (CNS). It is not known if this “photoreceptor signature” has any prognostic or therapeutic significance in retinoblastoma or other tumors of the CNS.
Human and mouse retinoblastomas have molecular, morphometric, neuroanatomic, and neurochemical features of interneurons, including amacrine and horizontal cells. Indeed, the cell-type specific features that are most common across human and mouse retinoblastomas are those related to amacrine cell differentiation. As previously discussed, the tumor’s cell of origin may be completely independent of amacrine interneurons, and because tumor suppressor or oncogenic pathways are deregulated during tumorigenesis, the amacrine differentiation program may be aberrantly activated during retinoblastoma tumorigenesis.
Nonetheless, the amacrine differentiation of human and mouse retinoblastoma may be functionally significant for tumor survival and progression. The most striking evidence for amacrine differentiation at the cellular level came from the identification of dense core vesicles in human and mouse retinoblastomas in electron micrographs. Retinoblastomas produce catecholamines and other monoamines, and several of the receptors for monoamines are expressed, as are the genes involved in the biosynthesis of monoamines. When monoamine receptors were blocked with pharmacologic agents, retinoblastoma growth was reduced in culture and in vivo. In contrast, agents that block the other major neurotransmitter signaling pathways (γ-aminobutyric acid, glycine, and glutamate) had no effect on retinoblastoma, nor did the inhibition of selective reuptake of catecholamines with pharmacologic agents. One interpretation of these data is that retinoblastoma cells are engaged in an autocrine induction of the catecholamine-signaling pathway. All of the major catecholamine/monoamine receptors are G-protein–coupled receptors and signal through adenyl cyclase–mediated cyclic adenosine monophosphate induction. This pathway can directly or indirectly stimulate the mitogen-activated protein kinase pathway and provide an important mitogenic signal for retinoblastoma. It may also explain why retinoblastomas form in the retina. That is, this tissue is uniquely susceptible to tumorigenesis because the amacrine-differentiation pathway can be co-opted for mitogenic signaling after RB1 gene inactivation. Additional studies will be required to determine whether catecholamine signaling influences survival and growth through the mitogen-activated protein kinase pathway or if it is required for some other aspect of tumorigenesis.
Retinoblastoma Genomics and Epigenomics
Most retinoblastomas initiate with biallelic loss of the RB1 gene. RB1 inactivation confers limitless replicative potential to retinoblasts, and these preneoplastic cells can progress to retinoblastoma by acquiring additional somatic mutations that contribute to the acquisition of new cellular properties, including evasion of cell death and senescence, sustained angiogenesis, and activation growth–signaling pathways. Several different mechanisms have been proposed to explain the rapid progression of retinoblastoma after RB1 inactivation. In a series of studies using genetically engineered murine cells and immortalized human cells, it was shown that RB1 may play an important role in maintaining genomic stability. Thus in some cellular contexts, inactivation of the RB1 gene could lead to chromosome instability, allowing secondary and tertiary mutations in key cancer pathways to be rapidly acquired. Alternatively RB1 has also been implicated in a variety of epigenetic processes, so it is also possible that perturbations in the epigenetic landscape may contribute to tumorigenesis in the retina. In support of an epigenetic mechanism, recent whole-genome sequencing and integrated epigenetic analysis of human retinoblastoma revealed that the tumors have relatively stable genomes, and several cancer genes were epigenetically deregulated. At least one of those epigenetically deregulated genes (spleen tyrosine kinase; SYK ) is required for retinoblastoma tumor cell survival in vivo (discussed later). These two alternative mechanisms (genome instability and epigenetic deregulation) of retinoblastoma progression are not necessarily mutually exclusive, and some tumors may show evidence of both chromosomal instability and epigenetic deregulation.
Although epigenetic deregulation of key cancer pathways has been shown to be directly relevant for retinoblastoma tumor progression, the functional significance of recurrent secondary genetic lesions is still poorly understood. During the 27 years since the RB1 gene was cloned, researchers have focused on identifying genetic lesions in retinoblastoma that contribute to tumor progression after RB1 inactivation. Cytogenetic and array comparative genome hybridization studies have led to the identification of regions of the genome that are gained or lost in retinoblastomas and may contribute to tumorigenesis. Those studies have led to the identification of candidate oncogenes and tumor suppressor genes whereby copy number variations (CNVs) correlate with changes in gene expression. However the major limitation of those studies is their relatively small number of tumors analyzed and the modest effects on gene expression for tumors with CNVs versus those without the corresponding lesion. Even for the most common recurrently mutated gene in retinoblastoma, BCL6 corepressor (BCOR) , there have been no direct in vivo studies showing that it contributes to tumorigenesis.
Carefully designed in vivo experiments to explore the role of putative oncogenes and tumor suppressor genes in retinoblastoma tumorigenesis are important for advancing our understanding of the molecular mechanisms of retinoblastoma progression. For example, a recent study showed that in a small proportion (1.5%) of human unilateral nonfamilial retinoblastomas, no RB1 gene mutations are present and they have v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN) amplification (≥10 copies). The authors suggested that in those patients, MYCN amplification may initiate tumorigenesis. This observation was recently validated and extended in an independent study with a separate cohort of patients.
Recent whole-genome sequencing of human retinoblastomas and their matched germline DNA demonstrated that at least some retinoblastomas have relatively stable diploid genomes with few CNVs or somatic nucleotide variations, and thus genome instability may not be required for retinoblastoma progression. One of the most common misconceptions related to cancer genomics is that tumors with few CNVs have stable genomes and tumors with more CNVs have unstable genomes. Chromosome instability is a dynamic process that cannot be accurately measured at a single time point because it involves the acquisition of sequential chromosomal lesions over time. It is important to distinguish between such dynamic processes that reflect the continuous accumulation of genetic lesions versus more acute genomic events such as multiple chromosome trisomies as seen in hyperdiploid acute lymphoblastic leukemia (ALL), multiple chromosome loss as seen in hypodiploid ALL, and the more recently described process of chromothripsis. The best way to directly analyze chromosome instability is to sample the same tumor at multiple time points and perform a comprehensive analysis of the genetic landscape at each time point. Orthotopic xenografts of human retinoblastoma in immunocompromised mouse eyes have proved to be very useful for analysis of chromosome instability in retinoblastoma because the genomic landscape can be analyzed sequentially over time. Clearly this does not preclude the possibility that some retinoblastomas have unstable genomes, but genome instability is not a universal hallmark of RB1 -deficient retinoblastomas, nor is it required for rapid tumor progression.
In the most recent genomic analysis of retinoblastoma, single nucleotide polymorphism 6.0 analysis was performed on 94 human retinoblastomas and matched normal germline DNAs. These data were used to identify regions of loss of heterozygosity and copy number changes, including whole chromosomes, regional changes, and focal changes (<3 Mb). Specifically, the majority (70%) of retinoblastomas have relatively few (≤10 per tumor) chromosomal, regional, or focal CNVs. No correlation was found between the number of CNVs and the heritable or sporadic form of disease, nor was there any relationship between the type of RB1 mutation and the number of lesions. A much larger study will be required to determine if more subtle associations exist between the clinicopathologic features of retinoblastoma and the rate of CNVs.
The overall low rate of CNVs was consistent with the paucity of focal recurrent lesions in genes. As reported previously, inactivation of the RB1 gene and the BCOR gene were the most common deletion events, and amplification of the MYCN gene was the most common focal recurrent gain. In this most recent study, a new recurrent focal amplification of orthodenticle homeobox 2 (OTX2) was discovered in 3% of retinoblastomas. OTX2 is a homeobox gene that is involved in photoreceptor and retinal pigment epithelium development. Retinoblastomas express a variety of rod and cone photoreceptor genes, and in future studies, it will be important to determine if OTX2 plays a role in modulating the photoreceptor differentiation program in retinoblastoma.
One of the most important aspects of the study was analysis of the relationship between mutations in MYCN, BCOR, and OTX2 and the mechanism of RB1 inactivation by sequencing all 27 exons of RB1 in the cohort. In total, 10 tumors were identified that had no single nucleotide variations or insertions or deletions in the coding region of RB1 . Whole-genome sequencing on those 10 tumors and their matched normal germline DNA led to the identification of one tumor with a wild-type RB1 gene and MYCN amplification. That tumor sample showed robust RB1 nuclear protein expression, suggesting that retinoblastoma can initiate in the absence of RB1 mutation. Gene expression array analysis showed that the tumor with wild-type RB1 was indistinguishable from all other retinoblastomas with RB1 mutations. Those data confirm a previous report from the Gallie laboratory that a small subset of retinoblastomas (~1% to 2%) have wild-type RB1 . More importantly this larger more recent genomic analysis also identified three tumors that had focal chromothripsis, disrupting the RB1 gene and leading to loss of RB1 protein expression. The chromothriptic lesions are not detected by routine genetic analyses because all the exons are present in the genome. This is the first example of chromothripsis in the RB1 gene in human cancer and the first clear example of chromothripsis as a mechanism for tumor initiation in a well-defined developmental cancer—retinoblastoma. Whole-genome sequencing combined with break-apart fluorescence in situ hybridization analysis of the RB1 locus and RB1 immunohistochemistry (IHC) can be used to identify this unique subset of retinoblastoma tumors.
Retinoblastoma Preclinical Models
Mouse models of cancer have become increasingly important in the fields of cancer genetics and translational research. The importance of mouse models is particularly true for pediatric cancers because the patient population is relatively small and preclinical models are essential for validating the efficacy of new combinations of chemotherapy before initiating clinical trials in children. In addition, animal models of cancer can provide important new insight into the molecular, cellular, and genetic mechanisms underlying tumor initiation and progression.
One challenge of modeling pediatric cancer in mice is that these tumors initiate in the context of developing tissues that change rapidly, and the cells that give rise to the tumors can display an extraordinary degree of plasticity and heterogeneity. This situation is further complicated by the fact that many tumor suppressor genes and protooncogenes play essential roles in regulating cell-fate specification and differentiation during development. Specifically, the genetic lesions that contribute to tumor initiation and progression may also alter the intrinsic cell-fate specification and differentiation programs in the tumor cells, thereby making it very difficult to infer the cell of origin for that tumor.
Retinoblastoma is a relatively simple tumor that initiates with a common genetic lesion ( RB1 inactivation) and progresses rapidly in children. Moreover many features of retinal development are also conserved across species, making retinoblastoma an ideal tumor for cross-species comparison.
During the past decade a series of knockout mouse models of retinoblastoma have been generated by conditionally inactivating multiple Rb family members in the developing retina. Knockout mouse models of retinoblastoma have also been valuable for studying the contribution of other tumor suppressor pathways such as the p53 pathway and for testing novel therapeutic agents for the treatment of retinoblastoma. In one study six different strains of mice that developed retinoblastoma were analyzed side by side using the same retinal progenitor–specific Cre transgene ( Chx10-Cre ) to inactivate tumor suppressor genes in the developing retina. Histopathologic analysis, gene expression profiling, and morphometric, neuroanatomic, and neurochemical analyses showed that mouse retinoblastomas faithfully recapitulate the molecular and cellular features of human retinoblastomas. However whereas the timing of retinoblastoma initiation was indistinguishable across the six strains, tumor penetrance and the rate of progression varied dramatically. These data raise the possibility that the genetic and epigenetic changes that accompany human retinoblastoma progression may not be faithfully recapitulated in the mouse, despite the remarkable interspecies similarities at the molecular and cellular level.
To more directly assess the similarities and differences across species for retinoblastoma, a recent study analyzed the aneuploidy, CNVs, somatic nucleotide variations, and epigenetic landscape of murine retinoblastoma. Similar to human retinoblastoma, mouse tumors have a low rate of single-nucleotide variations in genes. However mouse retinoblastomas have a higher rate of aneuploidy and regional and focal copy number changes, and this is dependent on the genetic lesions that initiate tumorigenesis in the developing murine retina. In addition the epigenetic landscape in mouse retinoblastoma was significantly different from human tumors, and some pathways that are candidates for molecular targeted therapy for human retinoblastoma such as SYK or myeloid cell leukemia–1 are not deregulated in genetically engineered mouse models (GEMMs). Taken together these data suggest that important differences exist between mouse and human retinoblastomas with respect to the mechanism of tumor progression, and those differences can have significant implications for translational research to test the efficacy of novel therapies for this devastating childhood cancer.
In fact, preclinical testing of combination chemotherapy (etoposide, carboplatin, and vincristine) showed dramatic differences in response between GEMMs and human orthotopic xenografts; virtually all of the GEMMs were cured of their disease, whereas no improvement was seen in progression-free survival or overall survival in the orthotopic xenograft model. It is possible that such species-specific differences could be further amplified when testing molecular targeted therapeutics directed toward processes important for maintaining genomic stability or the epigenetic landscape of retinoblastoma or targeting pathways such as SYK/myeloid cell leukemia–1 that are not deregulated in murine retinoblastoma. A careful assessment of the pathways that are deregulated in murine retinoblastomas is essential to make an accurate evaluation of the efficacy of novel therapeutics in preclinical studies focused on selecting the most promising new drugs for testing in clinical trials.
Understanding the intra- and interspecies differences is paramount when choosing the right model for the study of retinoblastoma. Unlike orthotopic xenograft models, genetic mouse models present the advantage of understanding the developmental processes that support tumor formation arising from a single cell and provide an ideal tool for genetic characterization of the contribution of individual pathways in retinoblastoma progression. On the other hand, orthotopic xenografts from patients faithfully recapitulate the mechanisms by which gene deregulation occurs in the human disease and can be more accurate for testing novel therapeutics. As a result, orthotopic xenografts are an important tool for target identification and target validation during preclinical trials, and GEMMs are important for understanding tumor initiation and progression in the developing retina.
Retinoblastoma Translational Research
Worldwide retinoblastoma is diagnosed in approximately 8000 children each year, including 250 to 300 patients in the United States. In developed countries more than 90% of patients with localized retinoblastoma disease are cured using a combination of chemotherapy, focal therapies, surgery (enucleation), and radiation therapy. However many patients in developing countries present with advanced intraocular or metastatic disease. Although mortality is low with aggressive multimodal therapy, partial or full loss of vision occurs in approximately 50% of patients with advanced bilateral retinoblastoma. In addition, significant late effects of therapy occur, including facial deformities and increased incidence of secondary malignancies. A vigorous effort is now under way to develop targeted therapies to improve ocular salvage and vision preservation and reduce late effects of therapy without compromising therapeutic outcomes.
Major advances have recently been made in understanding the biology of retinoblastoma, including identification of several molecular targets such as the inhibition of the MDM4-p53 interaction, SYK, histone deacetylase (HDAC), and the B-cell lymphoma–2 (BCL2) family of proteins. MDM4 is overexpressed in most human retinoblastomas and has been shown to contribute to tumor formation by suppressing p53. Consequently specific inhibition of the MDM4-p53 interaction represents a promising drug target for retinoblastoma treatment. The small-molecule MDM2/MDM4 antagonist nutlin-3a has been shown to efficiently induce p53-mediated cell death in retinoblastoma cells and mouse models of retinoblastoma. Subsequent characterization of the genetic and epigenetic landscapes of retinoblastoma revealed profound increases in expression of the protooncogene SYK . Although SYK is not expressed in the normal human retina, it is upregulated in all retinoblastomas analyzed to date. SYK is also expressed at high levels in metastatic and posttreatment retinoblastomas (Brennan and Bahrami, unpublished data). Many small-molecule SYK inhibitors with diverse physiochemical properties are in development for rheumatoid arthritis and oncology applications, and some of these novel therapeutics may eventually benefit patients with retinoblastoma. Moreover, previous studies of SYK inhibition have implicated a number of downstream signaling molecules, including the BCL2 family of proteins, as mediators of the SYK survival signal. Thus small-molecule BCL2 inhibitors currently in development for other cancers, such as Obatoclax, ABT-737, and TW-37, may also prove to be effective therapeutic agents for retinoblastoma. Retinoblastomas are also highly sensitive to HDAC inhibition, and many HDAC inhibitors are currently in clinical trials.
Retinoblastoma is unique because several well-established routes of drug delivery exist that provide flexibility when considering novel therapeutics. Systemic routes can be used for some drugs but are typically limited by poor penetration across the blood-retinal barrier (BRB) and systemic toxicity concerns. Local ocular delivery routes include topical (transcorneal), periocular (transcleral), intravitreal (direct injection) and intraarterial infusion. However all routes are not amenable to all compounds because of the physiochemical property of the compounds, the effects on normal tissues, and the schedule of drug administration. Some chemotherapy drugs such as topotecan effectively cross the BRB, and equivalent intraocular pharmacokinetic (PK) profiles result from either systemic or local topotecan. Topotecan is routinely administered daily for 5 days in children with cancer, providing repeat tumor exposure in an outpatient setting. In contrast carboplatin and nutlin-3a cannot be administered systemically because of insufficient penetration of the BRB, and in these cases, subconjunctival injections have been a significantly more effective delivery route. However subconjunctival injections are only performed during examination with use of an anesthetic once every 3 weeks, precluding the opportunity for repeat dosing of the drug. Moreover reports of significant and dose-limiting local ocular toxicities of carboplatin have prevented widespread use of subconjunctival delivery for children with retinoblastoma. These studies highlight the importance of pharmacokinetics and toxicokinetics in drug-specific route selection for intraocular targets.
Because of the young age of the patient population and the relatively rarity of the disease, preclinical testing is a critical component of developing retinoblastoma therapeutics. A standardized approach for evaluating efficacy and safety using mouse genetic models of retinoblastoma and human orthotopic xenograft models has been developed, but the large number of potential compounds, routes, and formulations precludes preclinical testing of all possible combinations. Previous work suggests that, given the unique challenges of drug delivery to the eye, one way to streamline the candidate selection process is to determine which route and formulation achieve optimal PK before conducting preclinical studies. When administered using the routes and formulations that, according to PK studies, achieved target intraocular exposure, topotecan, nutlin-3a, and carboplatin were all efficacious. In contrast, despite promising in vitro cytotoxicity, subconjunctival delivery of a SYK inhibitor failed to provide efficacy in preclinical studies; this failure was related to insufficient exposure. However alternative formulations and routes of local delivery did improve PK compared with systemic delivery; intraocular concentration never reached levels required for cytotoxicity.
In conclusion, effective translational research for retinoblastoma is built on a foundation of outstanding basic research to advance our understanding of the pathways that are deregulated in this devastating childhood cancer. From there, suitable targets for therapy are identified, such as MDM4, HDAC, or SYK, and small molecules that disrupt signaling through those pathways are identified and tested for in vitro cellular effects. Next pharmacokinetic, pharmacodynamic, and toxicokinetic studies are combined with approaches to optimize formulation of the drugs for the different routes of delivery. Finally when suitable exposure is achieved with minimal toxicity, comprehensive preclinical testing using validated preclinical models is performed to compare efficacy of the new combination with current standard of care and other possible novel therapeutic approaches. Variations in schedule and integration with upfront standard of care therapy can also be tested at this stage to achieve an optimal route, dose, and schedule for a new retinoblastoma clinical trial.
Genetic Counseling for Families with Retinoblastoma
Retinoblastoma is a unique neoplasm in that the genetic form imparts a predisposition to developing tumor in an autosomal-dominant fashion with almost complete penetrance (85% to 95%). The majority of such children acquire the first mutation as a new germline mutation, with only 15% to 25% having a positive family history. However some families display an inheritance pattern characterized by reduced penetrance and expressivity. These low-penetrance retinoblastoma mutations either cause a reduction in the amount of normal protein produced or result in a partially functional mutant protein. An MDM2 polymorphism has also been shown to modify the clinical penetrance of the RB1 mutation, either by enhancing RB1 haploinsufficiency or by increasing resistance to p53-mediated apoptosis. Also the RB1 gene mutation can occur at a late stage of embryogenesis, resulting in a variable expression depending on the tissue, causing mosaicisms in 10% to 15% of the patients or their progenitors. In general, however, based on the inheritance pattern and considering the existence of mosaicisms, the following risk estimates can be made, which may be refined on the basis of DNA sequence analysis of the RB1 gene in the patient with retinoblastoma and his or her offspring or siblings.
- 1.
Risk for offspring of survivors of retinoblastoma:
- a.
The risk of retinoblastoma arising in the offspring of survivors of bilateral disease is 45%.
- b.
In the case of patients with unilateral disease, investigators have estimated the risk of retinoblastoma overall to be 2.5%. However this estimate includes offspring of survivors of unilateral retinoblastoma who have a positive family history and whose risk is similar to that of bilateral cases—45%. If the family history is negative and genetic screening has not been performed, the actual risk is probably less than 2%.
- a.
- 2.
Risk for siblings of patients with retinoblastoma: In the case of siblings of bilaterally affected children whose parents are also affected, the risk of developing retinoblastoma is 45%; if the sibling is unilaterally affected, the risk is 30%. For cases without a family history, the empirically derived risk is 2% for siblings of bilateral cases and 1% for siblings of unilateral cases.
Genetic counseling is of the utmost importance to assist parents in understanding the genetic consequences of each form of retinoblastoma and to estimate the risk in relatives. Counseling is relatively straightforward when a parent is affected, or when the child presents with bilateral disease; these patients all have the heritable form. For children without an affected parent who have only a single tumor, there is always a question about whether they carry the mutated gene. Children with unilateral disease who are older than 2 years at the time of diagnosis are not likely to be gene carriers, but we recommend that all children with retinoblastoma be screened for mutations in RB1, regardless of laterality and age at diagnosis. With the refinement in methods of mutational analysis during the past decade, detection rates have increased from 20% to 30%, to 70% to 80%, to greater than 90% at present. Given the heterogeneity in the site and type of gene defects, no single technology will be sensitive and effective, and a multistep approach must be taken. More than 80% of the mutations can be detected with sequencing of the 27 exons of the RB1 gene using a quantitative multiplex polymerase chain reaction. However 10% to 20% of the defects are due to large deletions, and therefore deletion scanning and Southern blotting is required for cases with no detectable mutations by quantitative multiplex polymerase chain reaction. Finally a small proportion of cases (probably less than 5%) may result from gene inactivation by promoter methylation, and therefore screening for constitutional methylation should be considered if the other methods do not reveal a mutation. Finally 1% to 2% of retinoblastomas have germline RB1, and in these cases MYCN amplification and chromothripsis of the RB1 gene may be the initiating events.
With the recent improvements in the detection rates, genetic testing could be performed in the offspring of retinoblastoma survivors who are known to have the mutated gene (detection of the mutation is relatively easily accomplished if the parental mutation is known), and screening can be tailored appropriately. Even if genetic screening is negative for the mutation, newborn siblings of children with retinoblastoma should be examined periodically until they are about 2 years of age, because inherited retinoblastoma would be extremely rare beyond that age. In the future if the false-negative rate is negligible, one might expect that molecular diagnosis of mutations would lead to earlier treatment and better health outcomes for patients with retinoblastoma, with lower cost than conventional surveillance for children at risk.
Pathology of Retinoblastoma
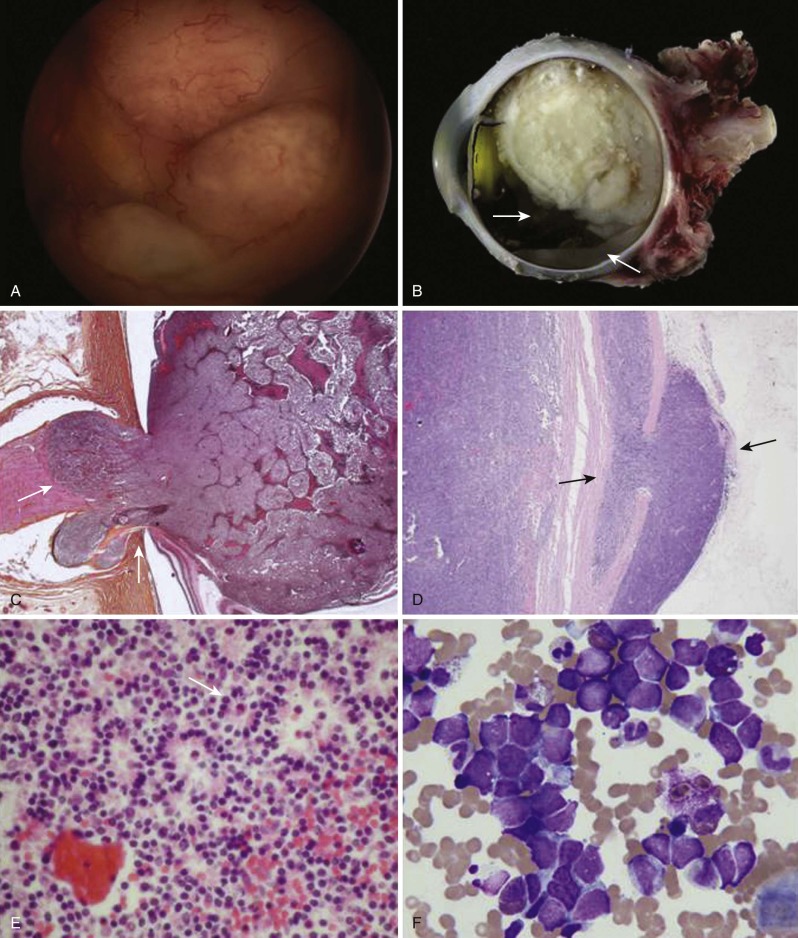
Retinoblastoma arises from the photoreceptor elements of the inner layer of the retina and usually extends into the vitreous cavity as a fleshy nodular mass (endophytic retinoblastoma). Less frequently it extends externally, causing a secondary retinal detachment, in which case there is no localized visible vitreous nodule (exophytic retinoblastoma). Macroscopically retinoblastoma is soft and friable and it tends to outgrow its blood supply, with resulting necrosis and calcification. Because of its friability, dissemination within the vitreous and retina in the form of small, white nodules (seeds) is common. In those cases it may be difficult to distinguish a multicentric primary tumor from a disseminated tumor.
Microscopically the appearance of retinoblastoma depends on the degree of differentiation. Undifferentiated retinoblastoma is composed of small, round, densely packed cells with hypochromatic nuclei and scant cytoplasm. Several degrees of photoreceptor differentiation have been described and are characterized by distinctive arrangements of tumor cells. Homer-Wright rosettes are composed of irregular circlets of tumor cells arranged around a tangle of fibrils with no lumen or internal limiting membrane. They are infrequently seen in retinoblastoma and are most often seen in other neuroblastic tumors such as neuroblastoma and medulloblastoma. Flexner-Wintersteiner rosettes, on the other hand, are specific for retinoblastoma. These structures consist of a cluster of low columnar cells arranged around a central lumen that is bounded by an eosinophilic membrane analogous to the external membrane of the normal retina. The lumen contains an acid mucopolysaccharide similar to that found around normal rods and cones. These rosettes are seen in 70% of tumors. The fleurettes are less often seen. In this case the cells exhibit even more ultrastructural characteristics of photoreceptor differentiation. They are composed of larger cells with abundant eosinophilic cytoplasm arranged in a distinctive fleur de lis pattern. Especially well-differentiated tumors composed almost entirely of fleurettes have been called retinomas or retinocytomas. Ultrastructurally retinoblastoma cells also demonstrate photoreceptor differentiation with the presence of the 9-0 microtubule doublet pattern, abundant cytoplasmic microtubules, synaptic ribbons, and neurosecretory granules.
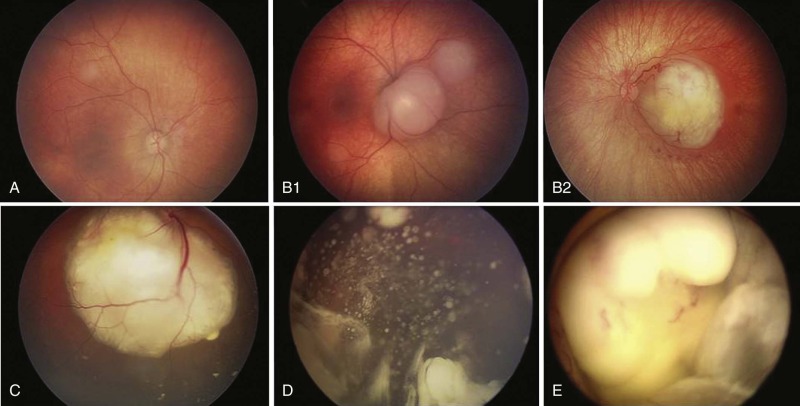
Dissemination of retinoblastoma occurs via several routes. Choroidal invasion provides access to a rich vascular network that serves as a potential route for distant metastases. In advanced cases direct extension occurs through the sclera into the orbit. Retinoblastoma can also invade the iris and the ciliary body and metastasize to the regional lymph nodes. Finally retinoblastoma can extend along the optic nerve, gaining access to the subarachnoid space and intracranial cavity. In cases of disseminated disease, positive cone-rod homeobox (CRX) staining may help differentiate retinoblastoma from other common small, blue, round cell malignancies of childhood. The typical macroscopic and microscopic characteristics of retinoblastoma are depicted in Figure 56-1 . Guidelines for processing and evaluation of the enucleated eye have been published.
Clinical Manifestations of Retinoblastoma
Retinoblastoma is by definition a tumor of the young child, and the age at presentation correlates with laterality. Patients with bilateral retinoblastoma tend to present at a younger age (usually before 1 year) than do patients with unilateral disease (often in the second or third year of life). Half of the cases of retinoblastoma diagnosed during the first year are bilateral, compared with fewer than 10% of cases diagnosed after 1 year of age. It is rare for retinoblastoma to be diagnosed during the first month of life, except in familial cases in which examination has been recommended early; however, regardless of the family history, in more than 90% of neonatal cases, either bilateral disease is present at presentation or asynchronous bilateral retinoblastoma will develop.
In more than half of cases the presenting sign is leukocoria, which occasionally is first noticed after a flash photograph ( Fig. 56-2 ). Strabismus, the second most common presenting sign, usually correlates with macular involvement. Very advanced intraocular tumors may become painful as a result of secondary glaucoma. Other childhood diseases that can present with leukocoria must be considered, such as persistent hyperplastic primary vitreous, retrolental fibrodysplasia, Coats disease, congenital cataracts, toxocariasis, and toxoplasmosis. In some series these nonmalignant conditions account for a significant proportion of enucleated eyes. In familial cases, the diagnosis is usually made through screening, although almost 50% of familial cases are diagnosed later in life, when patients present with the typical signs of retinoblastoma, underscoring the importance of genetic counseling.
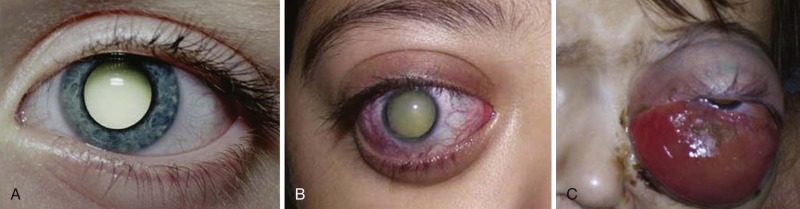
The successful management of retinoblastoma depends on the ability to detect the disease while it is still intraocular. Disease stage correlates with delay in diagnosis. In developing countries, late referrals are strongly associated with orbital and metastatic disease ( Fig. 56-2 ). For this reason, eye assessment should be performed in all newborns and at all subsequent health supervision visits by the primary care provider. Eye assessment is encouraged in all newborns and at subsequent health visits. The consensus guidelines by the American Academy of Pediatrics and Bright Futures for Pediatric Preventive Care include health supervision visits at birth and at 3 to 5 weeks of life, at 1, 2, 4, 6, 9, and 12 months during the first year, at 15, 18, and 24 months during the second year, and at 30 months and 3, 4, and 5 years of life. Importantly evaluation of vision and ocular health, including red reflex examination, is a component of each health supervision visit. Mass screening is also being considered, especially where the tumor presents in advanced stages, such as in areas of South America and Asia. Photoscreening is a system by which a photograph is produced by a calibrated camera under prescribed lighting conditions, which shows a red reflex in both pupils. A trained observer can identify ocular abnormalities by recognizing characteristic changes in the photographed pupillary reflex. This technique is fast, efficient, and reproducible, but it is still evolving.
Most patients with bilateral retinoblastoma carry a germline mutation of the RB1 gene. However a small proportion (5% to 6%) carry a deletion involving the 13q14 locus, which is large enough to be detected by karyotype analysis, either as a deletion or as part of a balanced translocation, most typically t(X;13). In those cases retinoblastoma is part of a more complex syndrome resulting from the loss of additional genetic material. Patients with the 13q deletion syndrome are characterized by typical facial dysmorphic features, subtle skeletal abnormalities, and different degrees of mental retardation and motor impairment. Dysmorphic features more consistently found include thick anteverted ear lobes, a high and broad forehead, a prominent philtrum, and a short nose. A proportion of patients also have overlapping fingers and toes, microcephaly, and delayed skeletal maturation. The severity of the deficits correlate with the size of the deletion; normal psychomotor development may be seen in patients in whom the deletion is restricted to the 13q14 band.
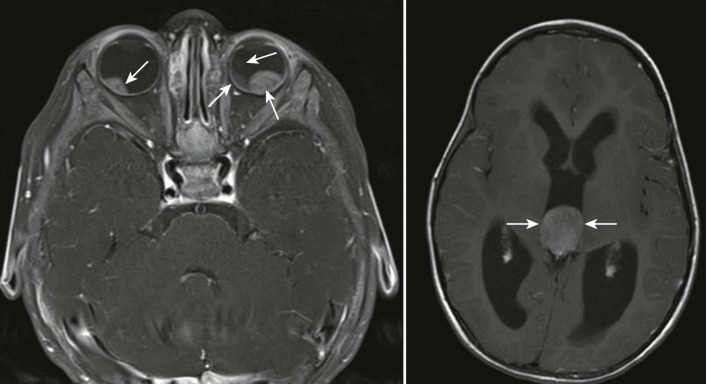
Trilateral retinoblastoma refers to the association of bilateral retinoblastoma with an asynchronous intracranial tumor ( Fig. 56-3 ). Tumors comprising trilateral retinoblastoma are primitive neuroectodermal tumors (PNETs) exhibiting varying degrees of neuronal or photoreceptor differentiation, suggesting an origin from the germinal layer of primitive cells. This association can occur in 3% to 9% of patients with the heritable form and appears to be more common in familial cases. Until recently the prognosis has been almost uniformly fatal; trilateral retinoblastoma has been the principal cause of death from retinoblastoma during the first decade of life in the United States. The majority of these tumors are pineal region PNETs (pineoblastomas), but in 20% to 25% of cases, the tumors are suprasellar or parasellar. Rare cases of quadrilateral retinoblastoma have been reported, in which bilateral retinoblastoma is associated with both pineal region and suprasellar intracranial primary PNETs. In most cases, the intracranial PNETs in association with retinoblastoma resemble undifferentiated retinoblastomas with the more frequent formation of Homer-Wright rosettes. The median age at diagnosis of trilateral retinoblastoma is 23 to 48 months, and the interval between the diagnosis of bilateral retinoblastoma and the diagnosis of the brain tumor is usually more than 20 months. Suprasellar tumors are usually diagnosed earlier, and in 15% to 20% of cases, the intracranial tumor antecedes the diagnosis of retinoblastoma. In recent years with the more widespread use of chemoreduction treatments and the decrease in the use of radiation therapy for patients with bilateral retinoblastoma, the incidence of trilateral retinoblastoma has decreased dramatically. However pineal cysts develop in approximately 5% to 8% of patients with bilateral disease; these cysts appear to be a forme fruste of trilateral retinoblastoma.
Diagnosis and Extent of Disease Evaluations in Retinoblastoma
The diagnosis of intraocular retinoblastoma is usually made without pathologic confirmation. An examination with use of an anesthetic with a maximally dilated pupil and scleral indentation is required to examine the entire retina. A careful examination of the iris and the anterior chamber is first performed, and the intraocular pressure is measured. Retinoblastoma usually appears as a mass projecting into the vitreous, although the presence of retinal detachment or vitreous hemorrhage may make its visualization difficult. Endophytic tumors are those that grow inward to the vitreous cavity. Because of its friability, endophytic retinoblastoma may seed the vitreous cavity ( Fig. 56-4, D ). Exophytic retinoblastoma grows into the subretinal space, thus causing progressive retinal detachment and subretinal seeding ( Fig. 56-4, C ). Exophytic tumors frequently resemble Coats disease. Less frequently retinoblastoma can adopt an infiltrative pattern without an obvious mass; this infiltrative pattern appears to be more frequent among older children. A very detailed documentation of the number, location, and size of tumors, the presence of retinal detachment and subretinal fluid, and the presence of vitreous and subretinal seeds must be performed. Wide-angle real-time retinal imaging systems such as RetCam (Clarity Medical Systems, Pleasanton, Calif.) provide a 130-degree field of view and digital recording, facilitating diagnosis and monitoring.
Additional imaging studies that aid in the diagnosis include bidimensional ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI). These imaging studies are particularly important to evaluate extraocular extension and to differentiate retinoblastoma from other causes of leukocoria. CT is very helpful to detect calcifications, although it is generally avoided to limit radiation exposure, particularly in children with bilateral disease, and MRI is very helpful in considering the differential diagnosis of Coats disease and other inflammatory conditions, along with persistent fetal vasculature of hyperplastic primary vitreous ( Fig. 56-3 ). In the absence of extraocular disease, MRI is not useful in the estimation of microscopic or optic nerve involvement.
Evaluation for the presence of metastatic disease also needs to be considered in a subgroup of patients. Metastatic disease occurs in approximately 10% to 15% of patients, and it usually occurs in association with distinct intraocular histologic features, such as massive choroidal and scleral invasion, or with involvement of the iris or ciliary body and optic nerve beyond the lamina cribrosa. In these cases additional staging procedures must be performed, including bone scintigraphy, bone marrow aspirates and biopsies, and lumbar puncture. In as many as one third of high-risk patients, the synthase of ganglioside GD2 messenger ribonucleic acid may be detected in the cerebrospinal fluid by reverse transcriptase-polymerase chain reaction, and it appears to correlate with massive involvement of the optic nerve, the presence of glaucoma at diagnosis, and a high risk of cerebrospinal fluid relapse. In general in the absence of high-risk disease in patients undergoing enucleation, and in patients with intraocular disease who are undergoing ocular salvage therapies, a metastatic workup is usually not necessary. In patients with extraocular disease the use of immunocytology with GD2 or CRX staining may increase the yield for detection of small clumps of metastatic cells.
Staging of Retinoblastoma
The Reese-Ellsworth (R-E) grouping system has been generally accepted as the standard for intraocular disease. This grouping system was initially designed to predict the outcome after external beam radiation therapy (EBRT). It divides eyes into five groups on the basis of the size, location, and number of lesions and the presence of vitreous seeding ( Box 56-1 ). However developments in the conservative management of intraocular retinoblastoma have made the R-E grouping system less predictable of eye salvage and less helpful in guiding treatment. A new staging system (International Classification of Retinoblastoma) has been developed, with the goal of providing a simpler, more user-friendly classification that is more applicable to current therapies. This new system is based on the extent of tumor seeding within the vitreous cavity and subretinal space rather than on tumor size and location, and it seems to be a better predictor of treatment success ( Box 56-2 ; Fig. 56-4 ).
Group I. Very Favorable
- Ia.
Solitary tumor smaller than 4 dd at or behind the equator
- Ib.
Multiple tumors, none larger than 4 dd, all at or behind the equator
Group II. Favorable
- IIa.
Solitary tumor, 4 to 10 dd, at or behind the equator
- IIb.
Multiple tumors, 4 to 10 dd, at or behind the equator
Group III. Doubtful
- IIIa.
Any lesion anterior to the equator
- IIIb.
Solitary tumor larger than 10 dd behind the equator
Group IV. Unfavorable
- Iva.
Multiple tumors, some larger than 10 dd
- IVb.
Any lesion extending anteriorly to the ora serrata
Group V. Very Unfavorable
- Va.
Massive tumors involving more than half the retina
- Vb.
Vitreous seeding
dd, Disk diameter.
Group A
Small Tumors Away from the Foveola and Disc
- •
Tumors ≤3 mm in the greatest dimension confined to the retina, and
- •
Located at least 3 mm from the foveola and 1.5 mm from the optic disc
Group B
All Remaining Tumors Confined to the Retina
- •
All other tumors confined to the retina not in group A
- •
Subretinal fluid (without subretinal seeding) ≤3 mm from the base of the tumor
Group C
Local Subretinal Fluid or Seeding
- •
Local subretinal fluid alone >3 to ≤6 mm from the tumor
- •
Vitreous seeding or subretinal seeding ≤3 mm from the tumor
Group D
Diffuse Subretinal Fluid or Seeding
- •
Subretinal fluid alone >6 mm from the tumor
- •
Vitreous seeding or subretinal seeding >3 mm from the tumor
Group E
Presence of Any or More of These Poor Prognosis Features
- •
More than two thirds of the globe is filled with tumor
- •
Tumor in the anterior segment
- •
Tumor in or on the ciliary body
- •
Iris neovascularization
- •
Neovascular glaucoma
- •
Opaque media from hemorrhage
- •
Tumor necrosis with aseptic orbital cellulitis
- •
Phthisis bulbi
For patients undergoing enucleation, pathologic staging that incorporates other features known to influence the modality of treatment and the prognosis, such as choroidal and scleral involvement, optic nerve extension, and the presence of metastatic disease, is used. Growth and invasion occur as a sequence of events, and extraretinal extension occurs only after the tumor has reached large intraocular dimensions. As part of this process, retinoblastoma extends into the ocular coats (choroid and sclera), the optic nerve, and the anterior segment. Extraocular disease is the next step in this progression; locoregional dissemination occurs by direct extension through the sclera into the orbital contents and preauricular lymph nodes, and extraorbital disease manifests as intracranial dissemination and hematogenous metastases. Different staging systems have been classically used, including the Grabowski-Adamson, the St. Jude Children’s Research Hospital, the American Joint Committee on Cancer (AJCC), and the International Retinoblastoma Staging System (IRSS). The IRSS is a newly proposed staging system developed by an international consortium of ophthalmologists and pediatric oncologists that incorporates the most important elements of the older systems ( Box 56-3 ). The IRSS ( Box 56-3 ) and AJCC ( Box 56-4 ) systems appear to be the most reliable for grouping patients according to their risk of extraocular relapse.
- Stage 0.
Patients treated conservatively
- Stage I.
Eye enucleated, completely resected histologically
- Stage II.
Eye enucleated, microscopic residual tumor
- Stage III.
Regional extension
- a.
Overt orbital disease
- b.
Preauricular or cervical lymph node extension
- a.
- Stage IV.
Metastatic disease
- a.
Hematogenous metastasis (without CNS involvement)
- 1.
Single lesion
- 2.
Multiple lesions
- 1.
- b.
CNS extension (with or without any other site of regional or metastatic disease)
- 1.
Prechiasmatic lesion
- 2.
CNS mass
- 3.
Leptomeningeal and CSF disease
- 1.
- a.
CNS, Central nervous system; CSF, cerebrospinal fluid.
Clinical Classification (cTNM)
Primary Tumor (T)
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
T1 Tumors no more than 2/3 the volume of the eye with no vitreous or subretinal seeding
T1a No tumor in either eye is greater than 3 mm in largest dimension or located closer than 1.5 mm to the optic nerve or fovea
T1b At least one tumor is greater than 3 mm in largest dimension or located closer than 1.5 mm to the optic nerve or fovea. No retinal detachment or subretinal fluid beyond 5 mm from the base of the tumor
T1c At least one tumor is greater than 3 mm in largest dimension or located closer than 1.5 mm to the optic nerve or fovea, with retinal detachment or subretinal fluid beyond 5 mm from the base of the tumor
T2 Tumors no more than 2/3 the volume of the eye with vitreous or subretinal seeding. Can have retinal detachment
T2a Focal vitreous and/or subretinal seeding of fine aggregates of tumor cells is present, but no large clumps or “snowballs” of tumor cells
T2b Massive vitreous and/or subretinal seeding is present, defined as diffuse clumps or “snowballs” of tumor cells
T3 Severe intraocular disease
T3a Tumor fills more than 2/3 of the eye
T3b One or more complications present, which may include tumor-associated neovascular or angle closure glaucoma, tumor extension into the anterior segment, hyphema, vitreous hemorrhage, or orbital cellulitis
T4 Extraocular disease detected by imaging studies
T4a Invasion of optic nerve
T4b Invasion into the orbit
T4c Intracranial extension not past chiasm
T4d Intracranial extension past chiasm
Regional Lymph Nodes (N)
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node involvement
N1 Regional lymph node involvement (preauricular, cervical, submandibular)
N2 Distant lymph node involvement
Metastasis (M)
M0 No metastasis
M1 Systemic metastasis
M1a Single lesion to sites other than CNS
M1b Multiple lesions to sites other than CNS
M1c Prechiasmatic CNS lesion(s)
M1d Postchiasmatic CNS lesion(s)
M1e Leptomeningeal and/or CSF involvement
Pathologic Classification (pTNM)
Primary Tumor (pT)
pTX Primary tumor cannot be assessed
pT0 No evidence of primary tumor
pT1 Tumor confined to eye with no optic nerve or choroidal invasion
pT2 Tumor with minimal optic nerve and/or choroidal invasion:
pT2a Tumor superficially invades optic nerve head but does not extend past lamina cribrosa or tumor exhibits focal choroidal invasion
pT2b Tumor superficially invades the optic nerve head but does not extend past lamina cribrosa and exhibits focal choroidal invasion
pT3 Tumor with significant optic nerve and/or choroidal invasion:
pT3a Tumor invades optic nerve past lamina cribrosa but not to surgical resection line or tumor exhibits massive choroidal invasion
pT3b Tumor invades optic nerve past lamina cribrosa but not to surgical resection line and exhibits massive choroidal invasion
pT4 Tumor invades optic nerve to resection line or exhibits extra-ocular extension elsewhere
pT4a Tumor invades optic nerve to resection line but no extra-ocular extension identified
pT4b Tumor invades optic nerve to resection line and extra-ocular extension identified
Regional Lymph Nodes (pN)
pNX Regional lymph nodes cannot be assessed
pN0 No regional lymph node involvement
pN1 Regional lymph node involvement (preauricular, cervical)
N2 Distant lymph node involvement
Metastasis (pM)
cM0 No metastasis
pM1 Metastasis to sites other than CNS
pM1a Single lesion
pM1b Multiple lesions
pM1c CNS metastasis
pM1d Discrete mass(es) without leptomeningeal and/or CSF involvement
pM1e Leptomeningeal and/or CSF involvement
CNS, Central nervous system; CSF, cerebrospinal fluid.
Although the clinical significance of extraocular extension is obvious, there is no uniform agreement on the prognostic implications of the different histologic characteristics, resulting in the use of different staging systems. Even in the absence of extraocular disease, a variable risk of developing metastatic disease exists. Many studies have attempted to evaluate the risk associated with the different histologic variables. Two thirds of patients have exclusive retinal disease, and invasion of the anterior segment, choroid, and optic nerve occur in variable proportions and combinations in the remaining patients. Extension of tumor into the sclera and across the line of transection of the optic nerve are associated with elevated mortality and by definition are considered to represent extraocular disease. The question arises when interpreting the other variables, because the risk associated with each variable is confounded by the lack of standardized grading methods and biased by the use of adjuvant chemotherapy.
Optic nerve involvement is common (occurring in 25% to 45% of all cases), but its impact on outcome appears to be limited to the involvement beyond the lamina cribrosa (where the meninges insert) and to the extension up to the transection line. The mortality rates for untreated patients with those features are 40% to 60% and greater than 80%, respectively.
Choroidal involvement is found in up to 40% of patients, although massive invasion occurs in fewer than 10% of cases. This distinction is important, because although choroidal invasion might have prognostic implications, its impact appears to be limited to cases with massive replacement by tumor. However contrary to optic nerve evaluation, criteria for determining the extent of choroidal disease are more subjective, and the grading of invasion is seldom reported. Consensus criteria for definition of massive or significant choroidal invasion include a maximum diameter (thickness or width) of invasive focus of tumor of 3 mm or more in any diameter and reaching the inner fibers of the sclera. A tumor focus of less than 3 mm and not reaching the sclera is considered focal choroidal invasion.
Evaluating the risk for each histologic variable individually is insufficient; different combinations of simultaneously occurring factors are very frequent. When choroidal invasion is present, half the cases will also show optic nerve involvement, whereas optic nerve invasion is uncommon (20%) if the choroid is intact. Conversely, when the optic nerve is invaded, 30% to 40% of cases will have choroidal replacement (more than 80% if the tumor has spread to the transection line), but significant choroidal invasion is quite rare (less than 20%) if the optic nerve is free of tumor. Therefore the risk associated with each histologic variable can only be estimated in the light of its association with others. Most available data support the notion that choroidal invasion alone is not associated with increased risk of extraocular spread, although massive choroidal replacement by tumor may be an exception. Similarly retrolaminar optic nerve invasion appears to be of prognostic significance only when there is concomitant significant choroidal invasion, but this represents fewer than 20% of cases. The significance of other histologic features, such as invasion of the anterior segment or grade of differentiation, remains unclear. The presence of extensive tumor necrosis appears to correlate with high-risk histologic features, such as postlaminar optic nerve involvement and choroidal invasion.
Clinical presentation correlates with disease only in cases of advanced intraocular disease; group E eyes, and those presenting with increased intraocular pressure or buphthalmos, have a higher frequency of high-risk histology.
Principles of Treatment of Retinoblastoma
Treatment of retinoblastoma aims to save life and preserve useful vision, and thus it needs to be individualized. Factors that need to be considered include laterality of the disease and RB1 germline status, potential for preserving vision, and intraocular and extraocular staging. For patients presenting with intraocular disease, particularly those with bilateral eye involvement, a conservative approach consisting of tumor reduction with intravenous or ophthalmic artery chemotherapy coupled with aggressive focal therapy may result in high ocular salvage rates. Radiation therapy, one of the most effective treatments in retinoblastoma, is usually reserved for cases of intraocular or extraocular disease progression.
Surgery
Enucleation is indicated for large tumors filling the vitreous for which there is little or no likelihood of restoring vision, and in cases of tumor present in the anterior chamber or in the presence of neovascular glaucoma. This corresponds to group E eyes and a significant proportion of group D eyes; upfront enucleation should be offered to those patients. Enucleation should be performed by an experienced ophthalmologist. The eye must be removed intact, without seeding the malignancy into the orbit and avoiding globe perforation. For optimal staging, a long section (10 to 15 mm) of the optic nerve needs to be removed with the globe. An orbital implant is usually fitted during the same procedure, and the extraocular muscles are attached to it. In the past, orbital implants were avoided because it was believed that they would interfere with the palpation of the socket and clinical detection of orbital recurrence. However with improved understanding of the histologic risk factors and the availability of better imaging techniques to detect orbital disease, implants should be placed at the time of the enucleation. Different orbital implants are available, including polymethylmethacrylate, polyethylene, and coralline and bovine hydroxyapatite spheres. A tissue wrap to the implant is placed, which will allow the four rectus muscles to be anatomically reattached, thus providing implant motility with little resistance in the orbit. The size and type of implant are important to stimulate orbital growth. A ceramic false eye is later fitted in the orbital socket. Orbital exenteration is very seldom indicated, although it should considered in cases of tumor recurrence after radiation. For patients presenting with orbital disease, a judicious use of chemotherapy, surgery, and radiation therapy will result in effective tumor control, avoiding the need for orbital exenteration.
Focal Therapies
Focal treatments are used for small tumors (less than 3 to 6 mm) and in combination with chemotherapy. In this setting of a patient undergoing chemotherapy for cytoreduction, focal treatments should be applied to tumors that fail to calcify; it is usually recommended to allow maximal reduction in tumor size with one or two cycles of chemotherapy prior to proceeding with aggressive focal consolidation to minimize the damage to the surrounding retina, particularly when the tumor is close to visually critical structures, such as the fovea and the optic nerve. The focal therapies available to treat retinoblastoma include argon green laser, diode laser, cryotherapy, and brachytherapy. The use of each modality depends on the tumor location and size. Lasers are more commonly used to treat posterior tumors, whereas more anterior tumors are easily accessed with cryotherapy. Brachytherapy is reserved for tumors that cannot be consolidated with either of those two approaches, usually because of their size.
Two wavelengths of light are used to treat retinoblastoma. The argon green laser, with a wavelength of 532 nm, photocoagulates tissue by inducing a temperature in excess of 65°C; it has been traditionally used to treat the retina edge surrounding a tumor in an effort to deprive the tumor of its blood supply and subsequently induce tumor regression, and for the treatment of retinal neovascularization due to radiation therapy. Using an indirect ophthalmoscope, a double row of white burns is used to encircle the tumor, and powers of 250 to 350 mW and burn durations of 0.3 to 0.5 seconds are used. This technique is limited to tumors measuring no greater than 4.5 mm in base and no greater than 2.5 mm in thickness. Debate exists as to whether the tumor surface should be directly treated for fear of releasing tumor cells into the vitreous. Newer generations of the 532-nm laser permit continuous delivery similar to that of the diode laser; such modalities have led some persons to advocate its use in the treatment of retinoblastoma. If the tumor surface is treated, the desired end point remains a subtle whitening of the treated areas. Laser energy should be kept at the lowest possible level necessary to achieve the desired end point; powers in excess of 500 mW will result in tumor disruption and should be avoided. As with all focal therapies, treatment should be continued until the residual fish-flesh tumor regresses into a flat chorioretinal scar. Typically treatment with lasers is administered every 3 to 4 weeks. The optimal indication for direct treatment with the argon green laser is a residual fish-flesh mass overlying a largely calcified tumor. In such situations, the uptake of the diode laser is unpredictable. With use of the argon green laser, a whitening of the tumor can be seen, ensuring an adequate treatment. The argon laser has both direct and indirect cytotoxic effects. Photocoagulation directly kills tumor cells, and the thermal spread from the laser may have secondary hyperthermic effects that act synergistically with ongoing chemotherapy. Argon laser photocoagulation should not be performed if there is a retinal detachment overlying the tumor.
The diode laser, with a wavelength of 810 nm, was adapted for the treatment of retinoblastoma based on its success in the treatment of choroidal malignant melanoma. The laser energy is readily absorbed by melanin pigment and proved successful in the treatment of small melanocytic tumors. Low-power settings coupled with a large spot size (2 to 3 mm) and prolonged delivery achieved hyperthermia (45°C to 65°C) at subphotocoagulation temperatures. The end result was a sustained penetrant burn with direct cytotoxic effects. The term transpupillary thermotherapy , or TTT, was coined to describe this technique. The methods of TTT were subsequently adapted to treat retinoblastoma. The infrared laser is focused directly on the tumor surface using either an ophthalmic microscope adapter or a large spot size indirect ophthalmoscope adapter. When using the indirect ophthalmoscope for TTT, it is important to ensure that the correct adapter is being used, because adapters designed for diode photocoagulation will not provide a continuous wavelength delivery necessary for hyperthermia. For small tumors the underlying retinal pigment epithelium aids in absorption and transfer of the heat to the tumor. The desired end point is a subtle whitening of the tumor free of hemorrhage. Powers are titrated based on the tumor size and pigmentation of the underlying retinal pigment epithelium but should not exceed 600 mW when there is visible uptake by the surrounding retinal pigment epithelium. Tumors with significant elevation or residual elements that rest on adjacent calcified tumor do not readily whiten with TTT. Prolonged treatment sessions of 5 to 10 minutes using powers of 600 to 800 mW may be needed to achieve a therapeutic effect. Even with such prolonged treatment, a visible change in the tumor may not be seen until follow-up examination 3 to 4 weeks later. If available the argon green laser may provide a more effective treatment. The sequential administration of thermotherapy with carboplatin enhances the antitumor effect by increasing the platinum-DNA adducts, for which reason thermochemotherapy is becoming a very important component in the treatment of intraocular retinoblastoma.
Cryotherapy is used for the treatment of small equatorial and peripheral lesions, measuring no more than 3.5 mm in base and no more than 2 mm in thickness. Cryotherapy is directly cytotoxic, causing cell lysis by disruption of the cell membrane that occurs after the formation of cytoplasmic ice crystals. Nitrous oxide gas is used to deliver a transscleral freeze via a cryoprobe under direct visualization. The lesion to be treated is visualized with an indirect ophthalmoscope, and the scleral underlying the tumor is indented with a cryoprobe. A succession of three freezes with three intervening thaws is applied to the tumor. An “ice ball” should be seen to encompass the tumor and adjacent vitreous with each freeze. Treatment should be limited to the area of the tumor to minimize damage to adjacent normal retina. Multiple tumors may be treated at one examination, but extensive treatments should be avoided. Aggressive cryotherapy may cause serous retinal detachments and iatrogenic retinal breaks. Although each cryotherapy unit may vary, we target a freezing temperature of −70°C during treatment. Treatments should be repeated at 3- to 4-week intervals until the lesion regresses to a flat chorioretinal scar. Posterior tumors can also be treated with cryotherapy. The conjunctiva is opened in the quadrant of the tumor to be treated. The curved cryoprobe is passed along the curvature of the eye and the tumor is indented. Once the tumor has been identified, the triple freeze-thaw is applied. This “cut-down” cryotherapy is usually reserved for recurrent posterior tumors not amenable to laser treatment. Also in addition to its effect on tumor control, cryotherapy contributes to increase the intraocular penetration of chemotherapy agents, presumably through disruption of the blood-vitreous barrier. In general local control rates of 70% to 80% can be achieved. Complications of focal treatments include transient serous retinal detachment, retinal traction and tears, and localized fibrosis.
Chemotherapy
Chemotherapy is indicated in patients with extraocular disease, in the subgroup of patients with enucleated eyes with intraocular disease and high-risk histologic features, and in patients with bilateral disease in conjunction with aggressive focal therapies. Agents with documented efficacy include microtubule inhibitors (vincristine and paclitaxel), platinum compounds (cisplatin and carboplatin), topoisomerase II inhibitors (teniposide and etoposide), alkylating agents (cyclophosphamide and ifosfamide), anthracyclines (doxorubicin and idarubicin), and topoisomerase I inhibitors (topotecan). The first clinical experience with chemotherapy in the treatment of retinoblastoma was reported with the use of nitrogen mustard in 1953. Institutional experiences in the management of extraocular retinoblastoma were reported subsequently, usually applying regimens modeled after the treatment regimens for metastatic neuroblastoma. Since then the role of chemotherapy has continued to expand, and it is now a main component in the management of intraocular disease.
Ocular Pharmacokinetics
Application of new therapeutic possibilities for cancer treatment involves drug delivery in many forms, but ocular drug delivery is hampered by the barriers protecting the eye. The eye is protected from the xenobiotics of the bloodstream by blood-ocular barriers. The anterior blood-eye barrier (blood-aqueous barrier) is constituted by the endothelial cells in the uvea. This barrier limits access of hydrophilic drugs from plasma into the aqueous humor; however, inflammation may disrupt the integrity of this barrier, causing unlimited drug distribution to the anterior chamber. The posterior blood-eye barrier (BRB) is formed by the neural retina, the retinal pigment epithelium (RPE), and the tight walls of retinal capillaries. The inner border of the neural retina faces the vitreous, and the outer border is next to the RPE. The neural retina is composed of nine layers, and the RPE is composed of a monolayer of polarized cells. The blood supply to the inner two thirds of the retina is from retinal vessels; the retinal endothelial cells have basal lamina, and they are surrounded by pericytes, thus forming tight junctions. The blood supply to the outer third of the retina and the RPE comes from the choroidal circulation. Unlike the retinal capillaries, the vasculature of the choroid has extensive blood flow and leaky walls; drugs easily gain access to the choroid extravascular space, but thereafter distribution into the retina is limited by the RPE and retinal endothelia. Lipophilicity, molecular weight, and protein binding (it is believed that only unbound drugs can pass through the BRB) are the main factors for penetration through the barrier. Inflammation and trauma also have important roles in breaking the BRB by disrupting tight junctions, increasing transendothelial vesicles, and increasing pinocytosis. It is possible that a similar phenomenon occurs in retinoblastoma.
Ocular Drug Transporters
The BRB restricts the movements of substances after systemic and periocular administration to the retina. The tight junctions form the penetration barrier to hydrophilic substances, thereby limiting the transfer of compounds both inward (blood to vitreous) and outward (vitreous to blood). The RPE has many important features essential for normal retinal function, such as transport of nutrients and waste products, regulation of ion, and fluid balance. RPE thus expresses many transporters and channels related to these physiologic functions. Among those the RPE and the endothelial cells also express efflux transporters such as P-glycoprotein, multidrug resistance–associated protein–1, –4, and –5, and breast cancer resistance protein. This efflux protein activity restricts movements of certain drugs to the retina. In most cases this is an important protective system for the retina, but it also limits drug delivery to the posterior eye segment. Many antineoplastic agents are known substrates of efflux proteins.
Mechanisms for Transscleral Drug Delivery
Transscleral drug delivery is a potential solution to the need to achieve high intraocular concentrations of chemotherapeutic agents. Traditionally subconjunctival, subtenon, and retrobulbar injections have been used to administer drugs such as steroids and local anesthetic agents. The high permeability of the sclera to macromolecules has revived the interest in this route of drug administration. For transscleral drug delivery, the drug is placed into a periocular space (subconjunctival, subtenon, peribulbar, retrobulbar, or posterior juxtascleral), and it may permeate from the periocular space into the vitreous via the anterior chamber, via a direct penetration pathway across the sclera, or through the systemic circulation. In the anterior chamber route, the drug reaches the aqueous humor either directly across the sclera and ciliary body or indirectly via the tear fluid and cornea, and it subsequently diffuses to the posterior chamber and into the vitreous. In the direct penetration pathway, the drug permeates into the vitreous through the underlying tissues; in the anterior eye, the drug may diffuse through the ciliary body and then to the posterior chamber and vitreous, whereas in the posterior eye, the drug has to permeate across the choroid, retinal pigment epithelium, and neural retina to reach the vitreous. Finally a small proportion of the drug enters the general circulation via conjunctival, episcleral of choroidal vessels and returns into the eye with the systemic blood flow. The relative importance of each route to vitreal drug delivery has been investigated in animal models. The direct penetration pathway is the most important route in the vitreal delivery of most compounds with widely differing physicochemical properties.
The drug must permeate across several tissues, and some of the barriers are different in the anterior and posterior eye. The RPE is the rate-limiting permeation barrier in the retinal delivery of hydrophilic drugs and macromolecules through the transscleral route. Permeability in RPE is determined both by physical-chemical factors (e.g., molecular weight, lipophilicity, and charges) and affinity to active transporters. The transporters may decrease or increase the drug transfer across the RPE depending on the type of transporter (influx or efflux) and its location (vitreal or blood side). The permeating drug also faces active barriers such as the clearance via choriocapillaris blood flow. The choroidal blood flow is higher than in any other tissue (relative to the tissue weight), and choriocapillaris walls contain large fenestrations. Large macromolecules are able to diffuse through the fenestrations in rabbit choriocapillaris. In a rabbit model for administration of triamcinolone acetonide, elimination of the blood flow with cryotherapy did not result in improved penetration of the drug after subtenon administration. The orbital and conjunctival vasculature and lymphatics seem to have a larger role in drug clearance than does the choroid. The elimination of conjunctival lymphatic and blood flow by incising a small window in the conjunctiva was more effective in increasing the vitreous concentration than was the elimination of the choroidal blood flow. When considering the orbital clearance, the posterior subtenon location appears to be the best periocular route, because it is closer to the sclera and farther from the orbital vasculature. After periocular injection, the drug must penetrate across the sclera, which is more permeable than the cornea. However for the drug to reach the retina, it must pass across the choroid and RPE (a tighter barrier than the sclera), at which point drugs may be cleared significantly to the bloodstream.
The sclera has a large surface area (16.3 cm 2 ), and scleral permeability shows no dependence on lipophilicity but dependence on molecular radius. The sclera is composed of collagen and proteoglycan fibers with few protein-binding sites. Drugs permeate through the aqueous intercellular media of the sclera, occupying the pores between the collagen fibers. Pore diameter and intercellular space are important determinants of transscleral drug delivery, particularly for drugs such as large hydrophilic peptides and oligonucleotides. Scleral permeability is affected by an increase in intraocular pressure. Scleral permeability may be enhanced in several ways. Treatment of many ocular diseases incorporates the use of transscleral depots, either as scleral implants (used for antiinflammatory agents) or collagen matrix and fibrin sealants, or the use of microspheres and other nanoparticles.
Periocular Chemotherapy in Retinoblastoma
Control of the intraocular tumor burden requires achieving high levels of chemotherapeutic agents in all the intraocular compartments, including the vitreous, the subretinal space, and the ocular coats. Although it is effective systemic administration of chemotherapy is limited by the blood-ocular barriers previously described. In recent years methods to increase the intraocular concentrations of chemotherapeutic agents have been extensively investigated, and periocular administration of carboplatin has been incorporated into most regimens for the treatment of advanced intraocular retinoblastoma. In the transgenic murine retinoblastoma treated with intravitreous injections of carboplatin, inhibition of tumor growth occurs in a dose-dependent manner, thus suggesting that higher intraocular levels of chemotherapy would be desirable. In this retinoblastoma model, subconjunctival administration of carboplatin resulted in tumor control in a dose-dependent manner, suggesting good intraocular penetration of this agent when given periocularly. Pharmacokinetic studies performed in the rabbit eye have validated this method. Mendelsohn and colleagues compared the intraocular concentrations of agents after local or systemic administration in primates. Intravenous carboplatin was given at the standard dose used in humans (18.7 mg/kg), and systemic and intraocular pharmacokinetics were compared with peribulbar and episcleral injections of 10 mg of carboplatin. The intravenous administration resulted in higher levels in the aqueous humor than did peribulbar or episcleral injections. However vitreous levels were significantly higher when carboplatin was administered periocularly. Importantly systemic absorption after periocular administration was minimal; plasma levels of carboplatin were 3% of those achieved after intravenous administration. In the same study no etoposide was detected in the aqueous or vitreous humor when given systemically. Etoposide is a protein-bound drug, and it likely stays in the plasma and thus has limited penetration. Studies in the mouse model have also shown little intravitreal penetration of etoposide when given intravenously. The vitreous/plasma ratios when measured as area under the curve concentrations were 0.59 for carboplatin and 0.07 for etoposide. However these models may not recapitulate the natural history of retinoblastoma because the blood-vitreous barrier is likely disrupted by tumor. In fact intraocular carboplatin concentrations in the human eye with retinoblastoma are higher after intravenous administration than what the primate eye model shows; levels in the vitreous are much higher than what the animal studies would predict and are similar to levels measured in unaffected animals when the drug is given after cryotherapy. The periocular route has also been explored in animal models for topotecan and for new investigational agents such as SYK and MDM4 inhibitors.
Given the promising preclinical data, periocular administration of carboplatin has been progressively incorporated into the clinical practice. Patients tolerate the administration of 2 mL of the 10 mg/mL solution, although acute orbital toxicity is significant. In the only phase II study reported, responses were seen in three of the five eyes with vitreous disease and in two of the five eyes with retinal tumors. The role of this form of administration in the management of retinoblastoma is still being evaluated. Patients with advanced intraocular disease (particularly those with massive vitreous or subretinal seeding) are candidates to receive up to three doses of periocular carboplatin concomitantly with systemic chemotherapy to maximize intraocular levels of the agent. However great caution should be exerted if periocular carboplatin is administered; acute toxicities (e.g., periorbital edema and inflammation) and long-term toxicities (e.g., orbital fat necrosis, soft tissue and muscle fibrosis, and optic neuritis) are common. Although periocular carboplatin has a very limited role as a single agent, it may lead to long-term disease control when combined with other treatment modalities ( Box 56-4 ). Topotecan has also been used via periocular administration, although its intraocular penetration may be lower and its potential role in therapy may be more limited. In the animal model periocular administration of topotecan reached potentially active levels of its active lactone moiety without significant toxicity. However systemic absorption was significant. A recently completed phase I study has documented the feasibility of administering 2 mg of topotecan periocularly, although no significant responses were seen.
Although effective in increasing intraocular levels, subconjunctival administration of carboplatin or topotecan in aqueous media has many limitations. Most of the drug delivered is dispensed quickly throughout the subconjunctival space and surrounding orbit, thus exposing extraocular tissues to high transient concentrations of the drugs and potential toxicities. Methods for improving periocular delivery while minimizing toxicity include the use of a collagen matrix or fibrin sealants. Studies have shown that carboplatin may enhance fibrin clot formation. One benefit of the sealant is that the anhydrous form of carboplatin can be overloaded beyond its solubility limit to provide higher levels of drug for extended periods. The scleral drug exposure is maximized, extending the duration of therapeutically significant intraocular drug penetration, and decreasing the need for repeated periocular injections. However in the animal models, as the intraocular levels increase, so does the risk of retinal toxicity.
Intravitreal and Intraarterial Chemotherapy for Intraocular Retinoblastoma
Administration of chemotherapy drugs directly into the tumor vasculature is a common practice in oncology. A small, localized tumor such as retinoblastoma would be an excellent candidate for such an approach. Japanese investigators have pioneered the administration of intravitreal and intraarterial melphalan for patients with advanced or recurrent intraocular retinoblastoma. Preclinical data suggest that retinoblastoma is very sensitive to melphalan and that there is a synergistic effect with thermotherapy and with other agents such as topotecan. Direct delivery to the ocular vasculature has been limited by the technical difficulties in the cannulation of the retinal artery. Kaneko and Suzuki initially reported the feasibility of injecting melphalan into the ipsilateral carotid artery, with documented efficacy. The technique was later perfected by Suzuki and colleagues and Mohri using a balloon catheter that allowed for selective injection into the ophthalmic artery. More recently Abramson and colleagues reported a variation of this technique that includes a direct cannulation of the ophthalmic artery using a microcatheter (supraselective). Successful catheterization and delivery of chemotherapy can be achieved in 98% of cases, although in 16% of patients an alternative vascular route is required—often the orbital branch of the middle meningeal artery. This approach has also been documented as being feasible as tandem therapy in patients with bilateral disease.
Although melphalan has remained the most commonly used agent and the most effective agent, it is often combined with topotecan or carboplatin when responses are suboptimal or the intraocular disease is very advanced. The doses of the chemotherapeutic agents are determined by patient age (and ocular volume) and by the angioanatomy; doses reported to result in a significant antitumor effect and limited toxicity are 2.7 to 7.5 mg for melphalan, 0.3 to 0.6 mg for topotecan, and 25 to 50 mg for carboplatin. In pigs, the infusion of 7 mg of melphalan or 1 mg of topotecan to the ophthalmic artery results in peak vitreous levels higher than the inhibitory concentration of 50%, and with limited systemic exposure. In children a large variability of systemic exposure occurs, and patients receiving tandem administrations of melphalan have a higher risk of the development of neutropenia, which correlates with plasma levels.
Patients typically receive one to six (median, three) intraarterial administrations of chemotherapy including melphalan alone or in combination, and responses are usually seen immediately after the first administration. For patients with treatment-naive eyes, the 2-year radiation-free ocular survival rate is 80% to 90%. Similar outcomes are also being reported with single-agent carboplatin and in combination with topotecan. Outcome correlates with the intraocular burden; patients with early intraocular disease (group B and C eyes) have an excellent outcome and may be treated with single-agent therapy. Eyes with significant vitreous or subretinal seeding have a worse outcome, with radiation-free survival rates of 80% for eyes with subretinal seeding and 65% for eyes with vitreous seeding. For patients with very advanced intraocular disease, an alternative is the use of systemic chemotherapy followed by consolidation with intraarterial melphalan. The presence of massive retinal detachment is not a contraindication to intraarterial chemotherapy; studies have shown that this approach promotes retinal reattachment and may result in ocular salvage rates in excess of 85%. Ocular salvage rates when intraarterial chemotherapy administration is used as salvage for patients with recurrent or progressive disease are consistently lower, with globe survival rates of 50% to 60%. However the use of a more intensive three-drug regimen with melphalan, topotecan, and carboplatin in patients whose disease has progressed after undergoing standard systemic and focal therapies has been reported to result in radiation-free ocular salvage rates of 75% at 2 years.
Patients with bilateral disease can receive tandem administrations. In those circumstances patients are at a higher risk of systemic toxicity as a result of melphalan exposure, and single-agent carboplatin may be used to treat the eye with less advanced disease during the tandem procedure. For neonates and very young infants for whom the cannulization of the ophthalmic artery is not feasible, a “bridge” treatment with single-agent systemic carboplatin until the baby is 3 months old or weighs 6 kg followed by consolidation with intraarterial chemotherapy has been shown to be very effective, with 1-year radiation-free ocular survival of 95%.
Direct administration of high doses of chemotherapy to a sensitive organ such as the eye of a young child is not devoid of complications. In the nonhuman primate model, treatment with three cycles of melphalan or carboplatin results in a significant inflammatory response, with leukostasis and retinal arteriole occlusion with ultrastructural changes in the endothelial cells and surrounding pericytes. Eyes also show nerve fiber infarcts and optic nerve hemorrhage and leukostasis, central retinal artery thrombosis, choroidal inflammation, and birefringent intravascular foreign bodies. Similar histopathologic evidence of vasculopathy is seen in enucleated eyes of children with retinoblastoma after intraarterial chemotherapy. Clinically a transient chorioretinal ischemia has been documented during the procedure, the impact of which is not fully understood. However approximately 15% of the eyes show a long-term sectorial choroidal atrophy, and 15% have delayed vitreous hemorrhage leading to enucleation. The impact of the intraocular vascular changes on vision has not been fully assessed because of the young age of the first cohorts of patients treated. The majority of patients do not have substantial electroretinographic changes, and preservation of central vision has been reported. However in patients with heavily pretreated eyes, intensive intraarterial chemotherapy may result in worsening of retinal function.
Acute and usually transient adverse events include mild eye edema, blepharoptosis, and orbital congestion with temporary dysmotility. Systemic effects are minimal; grade III neutropenia develops in only 10% of patients, although this proportion is higher in children with bilateral disease who undergo tandem therapy. Major vascular complications are very rare; no strokes or significant acute neurologic events have been reported when the procedure is performed at a state of the art treatment center. However stenosis of the ophthalmic artery and occlusion of the retinal artery have been documented. The risk of thrombosis is significantly increased in children with thrombophilia.
One additional risk associated with intraarterial chemotherapy is the exposure to ionizing radiation during fluoroscopy. In very experienced hands, the mean fluoroscopy time per procedure is 7 minutes. Radiation doses per procedure can be as high as 191 mGy to the affected eye and 35 mGy to the contralateral eye, although doses as low as 1 mGy per procedure have been reported by the center with the most experience in performing the procedure. After multiple procedures, cumulative doses can reach 0.1 to 0.2 Gy, which can be cataractogenic and potentially carcinogenic in this susceptible population. Long-term outcome data reported by Japanese investigators seem to indicate that no increase occurs in the incidence of second malignancies ; however, longer follow-up will be required to fully ascertain the risks associated with the procedure.
To achieve the maximum concentrations of chemotherapy close to the tumor and in the vitreous, investigators have attempted direct intravitreal delivery. This approach was pioneered by Swedish investigators in the 1960s. Thiotepa was the agent selected in those initial studies, and repeated injections of 1 to 1.5 mg were administered, often in conjunction with EBRT. Regressions were obtained, but long-term control of the intraocular disease was generally not possible. This approach was rekindled by Seregard et al. in three patients with bilateral disease with recurrent retinoblastoma in a single remaining eye. Patients received repeated intravitreal injections of thiotepa, followed by vitrectomy and weekly injections, for a total cumulative dose of 10 to 14 mg of thiotepa; no obvious clinical responses were observed.
Direct administration of melphalan into the vitreous is a promising approach for patients with vitreous disease. Clinical responses in patients with progressive retinoblastoma were obtained with use of intravitreal melphalan followed by hyperthermia, and more recent data seem to indicate that intravitreal chemotherapy may have a role in the management of patients with progressive vitreous disease. This approach is currently being explored in combination with systemic chemotherapy for patients with advanced intraocular disease.
Radiotherapy
Retinoblastoma is a very radiosensitive tumor. Radiotherapy in combination with focal treatments can provide excellent tumor control. However because radiation therapy increases the risk of second malignancies, contemporary management of intraocular retinoblastoma is designed to avoid or delay its use. The role of irradiation is mainly as salvage management for eyes that have failed to respond to chemotherapy and focal treatments, usually because of progression of vitreous and subretinal seeding. Because most patients with intraocular retinoblastoma who undergo radiotherapy have multifocal disease, the entire retinal surface needs to be irradiated to a uniform dose. Several techniques can be used, usually through lateral or anterior fields. Recommended total doses are 40 to 45 Gy, in 180 to 200 cGy fractions, although doses of 36 Gy and even lower may be effective in conjunction with other techniques. Radioactive plaque technique is useful when treating localized tumors, both because the procedure time is short and because a high dose of irradiation is delivered to the areas of interest while minimizing irradiation effects in extraocular structures.
Episcleral Plaque Brachytherapy
Indications for plaque therapy include solitary tumors with a diameter ranging between 6 and 15 mm, tumor thickness of 10 mm or less, and location of the lesion more than 3 mm from the optic disc or fovea. Since the early use of cobalt-60 plaques in the 1950s, the field has evolved to the more common use of iodine-125, iridium-192, and ruthenium-106. In combination with appropriate use of chemotherapy and focal consolidation, brachytherapy can be very effective, even in the presence of focal vitreous seeding overlying the tumor. The most commonly used radioisotope is 125 iodine, a low-energy γ-ray emitter (27 to 35 keV; half-life, 60 days) that is readily available in encapsulated form, allowing custom fabrication of gold plaques of varying sizes. 125 Iodine seeds are loaded within the wells of the plaque to achieve the dose and distribution desired based on the size and location of the tumor; lesions of up to 10 to 12 mm in height and 10 to 15 mm in diameter can be treated using this approach. 192 Iridium and 106 ruthenium are alternative isotopes of higher energy (295 to 612vkeV and 3.5 MeV, respectively) and longer half-life (74 and 374 days, respectively), which makes dosimetry calculations and safety measures more complex. The dose is prescribed to reach the tumor apex and overlying localized vitreous seeds if present. For 125 iodine the target dose is usually 40 Gy at the apex at 40 to −80 cGy per hour; the dose at the tumor base may approximate 120 Gy. Tumor control using this approach may be close to 80%. In approximately 25% of the eyes, long-term complications may occur, including nonproliferative retinopathy, papillopathy, and nonproliferative maculopathy. Similar results are achieved with ruthenium.
External Beam Radiation Therapy
EBRT is commonly used as consolidation therapy in conjunction with chemotherapy for intraocular retinoblastoma when the disease is not responding well to conservative management, usually because of the presence of vitreous or subretinal seeding or progression of the retinal tumors, and in the management of extraocular disease. Traditionally the opposed “D” fields have been used to treat bilateral retinoblastoma, with the isocenter placed 2 to 3 mm behind the lens; with this approach, aimed at sparing the lens, the retina anterior to the equator may be relatively underdosed. Currently the use of conformal and intensity-modulated radiation therapy (IMRTs) can provide coverage of the entire retina and vitreous cavity while limiting the dose to the lacrimal gland and soft tissues and orbital bones. The standard EBRT dose is 40 to 45 Gy, delivered in 180 to 200 cGy fractions; doses higher than 45 Gy do not seem to be associated with an increased benefit. One study also showed the potential role of low-dose (26 Gy) EBRT to consolidate advanced intraocular retinoblastoma upon completion of chemotherapy, in the absence of disease progression. IMRT may be superior to three-dimensional (3D) conformal radiation therapy and conventional two-dimensional irradiation in terms of the dose delivered to normal orbital and extraorbital tissues. However although increasing the conformity of the treatment results in a sharp decline of the dose-volume curve at the higher doses, this gain still comes at the expense of increasing the volume of normal tissue that receives the lowest doses. Even with optimal IMRT planning, 50% of the orbit receives 50% of the prescribed dose. The use of fractionated stereotactic radiation therapy may allow for a significant decrease in the orbital volume exposed. Proton beam therapy allows for exquisite dosimetry, thus providing a theoretic advantage in sparing radiation exposure to orbital tissues. A proton beam has exquisite stopping power and may produce essentially no lateral scatter, and thus protons can be used to control tumors at any depth without the entrance and exit doses associated with photon beam irradiation that are responsible for the complications associated with radiation therapy in patients with retinoblastoma. In a study comparing protons with conformal therapy, electron therapy, and IMRT, protons had the best orbital bone-sparing dose distribution. Only 10% of the bone exceeded 5 Gy, compared with 25% for 3D–conformal radiation therapy electrons, 69% for IMRT, 41% for a single 3D lateral beam, 51% for a 3D anterolateral beam with a lens block, and 65% for a 3D anterolateral beam without a lens block. Very importantly preliminary long-term data seem to indicate that the cumulative incidence of second malignancies in the irradiated field is significantly lower with protons when compared with standard photon therapy.
Radiation therapy is indicated for extraocular retinoblastoma and after enucleation when high-risk features are present (e.g., transscleral involvement and involvement of the cut end of the optic nerve). The target volume is the orbit and may extend to include regional sites and lymph nodes based on clinical and imaging evidence of tumor extension. The dose typically used for treatment of the orbit or regional extraocular sites is 45 Gy. Radiation therapy may be indicated for patients with metastatic disease based on response to chemotherapy. In most treatment protocols, patients with hematogenous metastases or CNS involvement who achieve a complete response to induction chemotherapy do not undergo irradiation; however, patients with an incomplete response usually undergo irradiation at the completion of therapy, which often includes consolidation with high-dose chemotherapy and autologous hematopoietic stem-cell rescue. This sequence is also recommended for patients who require craniospinal irradiation. Based on age older patients who require craniospinal irradiation should receive 36 Gy to the neuraxis and 45 Gy to residual disease. Younger patients should receive at least 23.4 Gy to the neuraxis. Evidence suggests that clinically defined disease has limited microscopic tumor infiltration so that the clinical target volume surrounding the clinically identifiable residual tumor (gross tumor volume) may be as small as 5 mm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree