(1)
Department of Surgery, Dar A lAlafia Medical Company, Qatif, Saudi Arabia
16.1 Introduction
Sickle cell anemia is known to be associated with several widespread complications that can affect any part of the body.
Recently, it was shown that sickle cell anemia vasculopathy plays an important role in the pathogenesis of several of these complications and NO deficiency plays a major role in this.
Endothelial generation of NO in the body has several important functions including:
Vasodilatation
Anti-inflammatory
Antithrombotic
Deficiency of NO is an important contributor to the etiology of sickle cell anemia vasculopathy.
In patients with SCA, deficiency of NO may be due to the following:
Scavenging of NO by the heme of sickle hemoglobin released into the plasma by the hemolyzed RBCs
Scavenging of NO by vascular superoxide anion
Depletion of plasma arginine by arginase released by hemolyzed RBCs
The effect of endogenous NO inhibitors
Inactivation of tetrahydrobiopterin, a NO cofactor, thereby uncoupling endothelial NO synthase such that superoxide anion, rather than NO, is produced
Patients with SCA develop renal complications including:
Recurrent episodes of hematuria
Renal tubular abnormalities:
A concentrating defect
Impaired potassium excretion
Acidification defect
It is estimated that about 70 % of patients with sickle cell anemia may develop microalbuminuria.
It is also estimated that about 26 % of adult patients with sickle cell anemia develop sickle cell related kidney disease. This increases with age.
The renal complications of sickle cell anemia are variable and include:
Functional abnormalities
Gross anatomic abnormalities
The sickle cell anemia renal complications include:
Gross hematuria
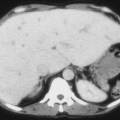
Renal infarction
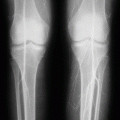
Papillary necrosis
Nephrotic syndrome
Urine concentration defects
Medullary kidney carcinoma
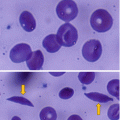
The renal medulla is relatively hypoxic, hypertonic, and acidotic. This predisposes to sickling of red blood cells (RBCs) leading to vaso-occlusion, which significantly decreases renal medullary blood flow.
Hematuria is commonly seen in patients with sickle cell nephropathy. It increases venous pressure, which further worsens ischemia of the renal medulla and predisposes the patient to further RBC sickling.
Proximal tubule dysfunction generally impairs urinary concentration, while more distal tubule dysfunction may impair potassium excretion, leading to hyperkalemia.
Renal Complications of Sickle Cell Anemia
Gross hematuria
Renal infarction
Papillary necrosis
Nephrotic syndrome
Urine concentration defects
Medullary kidney carcinoma
Hypertension is seen in only 2–6 % of patients with SCA. This is attributed to several factors including:
Sodium and water wasting secondary to pathological changes involving the renal medulla
Compensatory systemic vasodilatation due to significant reductions in the microcirculation and increased levels of prostaglandins and nitric oxide
There are several factors involved in the progression of renal complications to end-stage chronic renal failure. These include:
Worsening anemia
Hypertension
The degree of proteinuria
Microscopic hematuria
The Frequency of Renal Complications of Sickle Cell Anemia
About 26 % of adult patients with SCA develop sickle cell related kidney disease.
70 % of patients with SCA may develop microalbuminuria.
Hypertension is seen in only 2–6 % of patients with SCA.
About 4 % of patients with SCA develop renal failure at a median age of 23 years.
5–18 % of patients with SCA develop end-stage renal disease.
Proteinuria has been estimated to occur in 20–25 % of patients with SCA.
Renal infarcts and papillary necrosis occur in 30–40 % of patients with SCA.
Decreased renal function in 5–30 % of patients with SCA.
4 % of patients with SCA develop renal failure at a median age of 23 years.
Acute kidney injury occurs in 5–10 % of patients with SCA.
16.2 Pathophysiology
The renal medulla is characterized by:
Low oxygen tension.
Low pH.
High osmolality.
All of these predispose to RBC sickling, leading to increased blood viscosity which contributes to ischemia and eventual infarction that involves the renal microcirculation.
This will lead to medullary ischemia and infarction that cause papillary necrosis.
The sloughed papillae may obstruct urinary tract outflow, leading to renal failure.
It has been estimated that 5–18 % of patients with SCA develop end-stage renal disease.
The end-stage renal disease in patients with SCA is more likely to develop in patients with:
Hypertension
Proteinuria
Hematuria
Severe anemia
The recurrent bouts of hematuria and the renal tubular dysfunction arise secondary to renal medullary ischemia.
This is due to sludging of RBCs in the vasa recta.
This sludging of RBCs occurs because of:
Hypoxia
Hypertonicity
Acidosis in the medullary environment
A relatively low blood flow in the renal medulla
In patients with SCA, there is hypoperfusion of the renal medulla, while the cortex is often hyperperfused because of decreased renal vascular resistance.
This increased blood flow in the renal cortex leads to increased glomerular filtration rate.
An important adverse effect of hyperperfusion of the kidney is glomerulomegaly with subsequent development of glomerulosclerosis.
Glomerulosclerosis is the most common glomerulopathy in patients with SCA.
Renal infarcts and papillary necrosis occur in patients with sickle cell anemia with a prevalence of 30–40 %.
The prevalence of proteinuria among patients with sickle cell anemia has been estimated to be 20–25 %.
Patients with SCA have decreased kidney function which has been reported in 5–30 %.
About 4 % of patients with sickle cell anemia develop renal failure at a median age of 23 years. This is seen especially in patients with:
Hypertension
Proteinuria
Hematuria
Severe anemia
Hyperfiltration
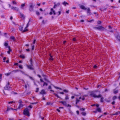
The histopathological changes seen in renal biopsies include:
Glomerular hypertrophy.
Hemosiderin deposits.
Focal areas of hemorrhage or necrosis.
Interstitial inflammation, edema, fibrosis, tubular atrophy, and papillary infarcts are seen in advanced stages.
Glomerular enlargement and focal segmental glomerulosclerosis (perihilar variant being most frequent) are seen in patients who reach end-stage renal disease due to sickle cell anemia.
The papillary necrosis associated with sickle cell anemia shows destruction of the vasa rectae and vascular congestion of the collecting ducts, inner medulla, and papillae.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree