Chapter 15 Red Blood Cell Membrane Disorders
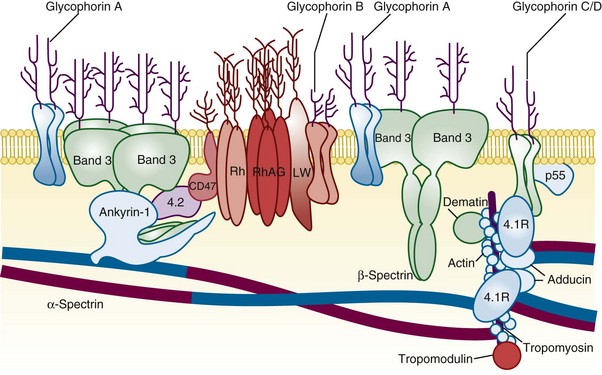
Figure 15-1 A SIMPLIFIED CROSS-SECTION OF THE ERYTHROCYTE MEMBRANE.
(From Perrotta S, Gallagher PG, Mohandas N: Hereditary spherocytosis. Lancet 372:1411, 2008.)
Table 15-1 Erythrocyte Membrane Abnormalities in Hereditary Spherocytosis, Hereditary Elliptocytosis, and Related Disorders
| Gene | Disorder | Comment |
|---|---|---|
| α-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. α-Spectrin mutations are most common cause of typical HE. |
| Ankyrin | HS | Most common cause of typical dominant HS. |
| Band 3 | HS, SAO, NIHF | In HS “pincer-like” spherocytes on smear presplenectomy. SAO erythrocytes have transverse ridge or longitudinal slit. |
| β-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. In HS, acanthrocytic spherocytes on smear presplenectomy. |
| Protein 4.2 | HS | Common in Japanese HS. |
| Protein 4.1 | HE | |
| Glycophorin C | HE | Concomitant protein 4.1 deficiency is basis of HE in glycophorin C defects. |
HE, Hereditary elliptocytosis; HPP, hereditary pyropoikilocytosis; HS, hereditary spherocytosis; NIHF, nonimmune hydrops fetalis; SAO, Southeast Asian ovalocytosis.
Table 15-2 Peripheral Blood Film Evaluation in a Patient With Red Cell Membrane Disorder
| Shape | Pathobiology | Diagnosis |
|---|---|---|
| Microspherocytes | Loss of membrane lipids leading to a reduction of surface area resulting from deficiencies of spectrin, ankyrin, or band 3 and protein 4.2 Removal of membrane material from antibody-coated red cells by macrophages Removal of membrane-associated Heinz bodies, with the adjacent membrane lipids, by the spleen | HS Immunohemolytic anemias Heinz body hemolytic anemias |
| Elliptocytes | Permanent red cell deformation resulting from a weakening of skeletal protein interactions (such as the spectrin dimer-dimer contact). This facilitates disruption of existing protein contacts during shear stress–induced elliptical deformation. Subsequently, new protein contacts are formed that stabilize elliptical shape Unknown |