Introduction
In summary, it appears that the tumor-filled ducts are a mixture of both cancer-filled preexisting ducts (in situ) and newly formed ducts (neoductgenesis) in this special subtype of breast cancer. The result is a conglomerate of “comedo carcinoma in situ” or Grade 3 DCIS combined with “highgrade duct forming invasive carcinoma”/“neoductgenesis.” Both will have central necrosis and often amorphous calcifications with solid or micropapillary cell architecture surrounded by a basement membrane. The new, cancerous branches of the ducts also have a distinct desmoplastic reaction and extensive lymphocytic infiltration and are often associated with neoangiogenesis.
The unique subgross histologic presentation of this breast cancer subtype helps us understand that this disease has the ability to disseminate both hematogenous and lymphatic metastases even in the absence of demonstrable “classic” invasive foci. The large, high-grade invasive tumor burden accounts for the poor long-term outcome, which is similar to that of the large and high-grade classical invasive tumors.
An additional problem is that the associated small focus (foci) of classical invasive, generally Grade 2 carcinoma is currently considered to be the determining component for the TNM classification of this breast cancer subtype. The small, often Grade 2 invasive foci fail to account for the frequent extensive lymph vessel invasion. Occasionally the lymph vessels contain cancer cells resembling the Grade 3 cells seen in the “DCIS” component, although the invasive focus is Grade 2.
Examples with Fatal Outcome
Example 5.1
A 51-year-old asymptomatic woman, screening examination. She was called back for assessment of the microcalcifications found on the screening mammograms of the right breast.
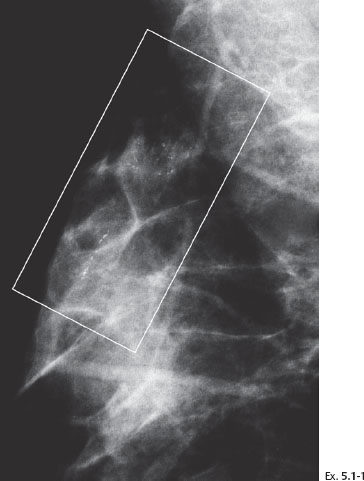
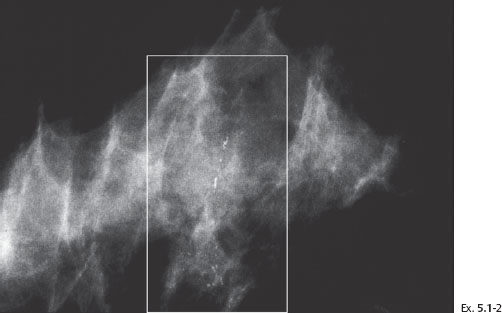
Example 5.1
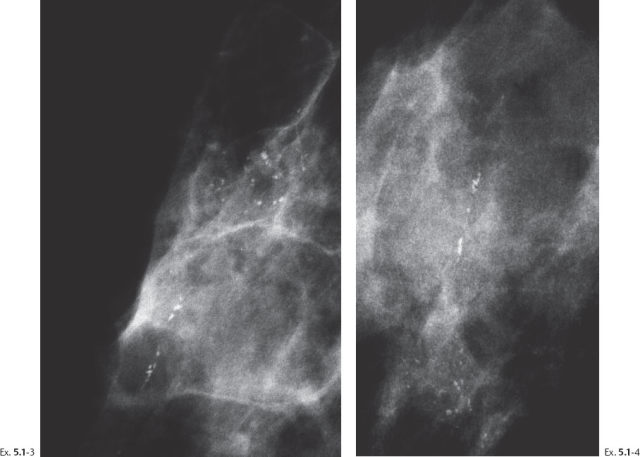
Ex. 5.1-3 & 4 Microfocus magnification views of the areas in the rectangles in Ex. 5.1-1 & 2, MLO and CC projections. The mammographically malignant type calcifications are a mixture of crushed stone–like and casting type calcifications.
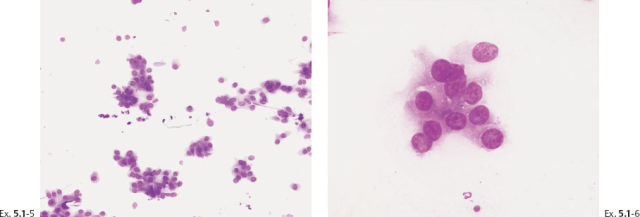
Ex. 5.1-5 & 6 Preoperative FNAB under stereotactic guidance shows malignant cells.
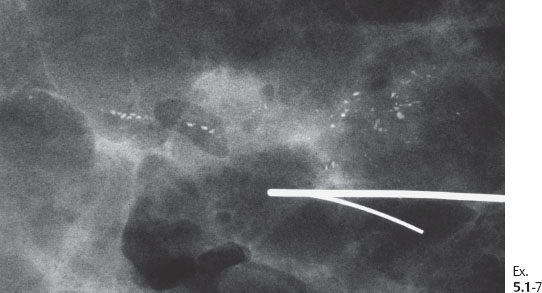
Ex. 5.1-7 Specimen radiograph: the microcalcifications have been removed with a good margin.
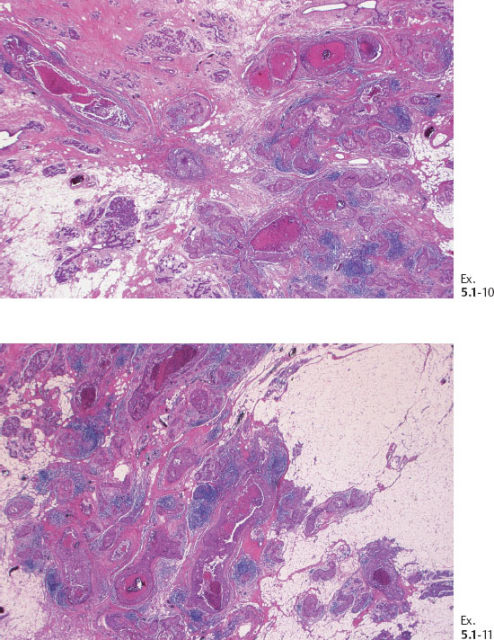
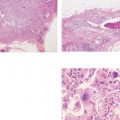
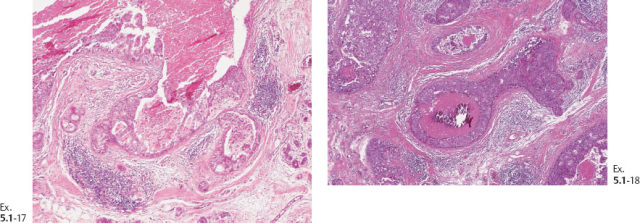
Ex. 5.1-10 & 11 Medium-power histological image. Cross section of a large number of cancerous ducts, which are spaced unusually close together, surrounded by a desmoplastic reaction and lymphatic infiltration.
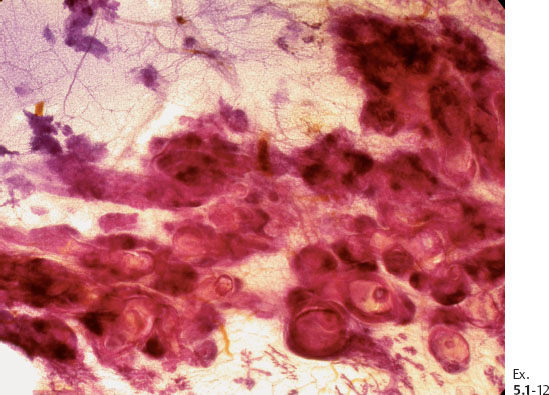
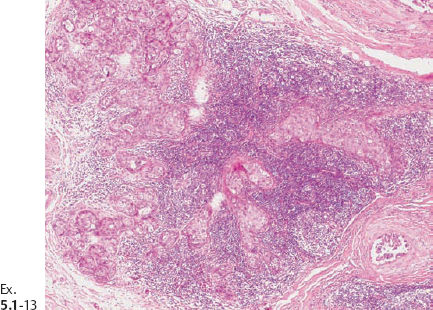
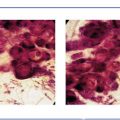
Ex. 5.1-13 A strong lymphocytic reaction surrounds the cancerous process.
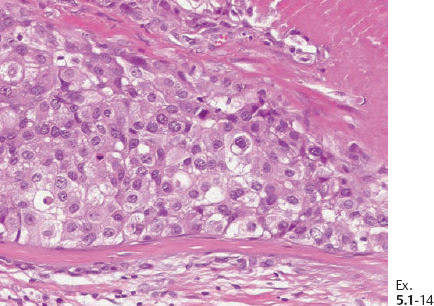
Ex. 5.1-14 Grade 3 cancer cells, solid cell architecture.
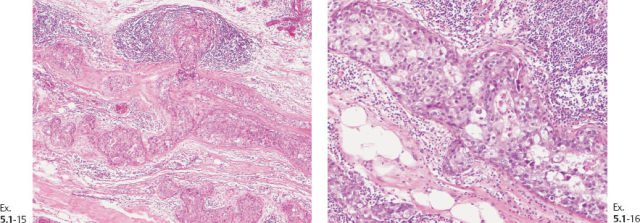
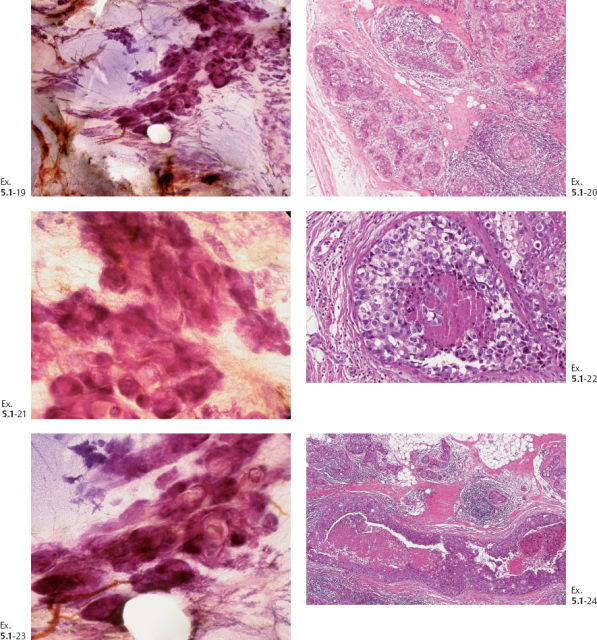
Ex. 5.1-19 to 24 Comparative subgross, 3D histological images (19, 21, 23) and conventional histology (20, 22, 24), demonstrating the abnormal ductlike structures distended by cancer cells, necrosis, and occasional amorphous calcifications.
Interpretation: According to conventional histological criteria, the presence of a basement membrane classifies these malignancies as an in-situ process, “DCIS.” However, many of the ducts have a dense and disorganized architecture and show tenascin overexpression, lymphocytic infiltration, and a desmoplastic reaction, all of which indicate that these particular ducts are newly produced during tumor growth and penetrate into the surrounding tissue. Our interpretation is that Grade 3 “DCIS” with castings over a large area has invasive roperties (neoductgenesis), which can account for the fatal outcome in this case, since we must assume that classic in-situ carcinoma, if it be truly in situ, could not have been the cause of death.
Follow up: Yearly follow-up with clinical breast examination and mammography showed no abnormality until six and one-half years after sector resection and radiotherapy, when the patient was hospitalized because of histologically proven breast cancer metastases to the lungs and skeleton (Ex. 5.1-25 to 30).
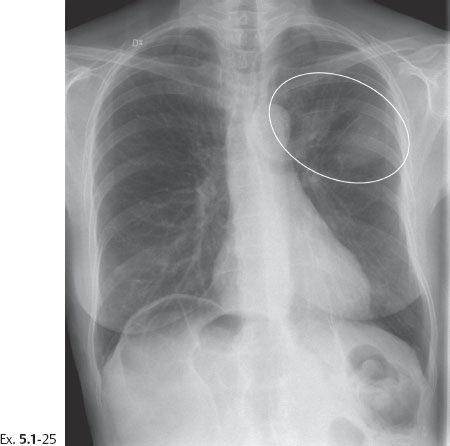
Ex. 5.1-25 Chest radiograph showing densities suspicious for metastases.
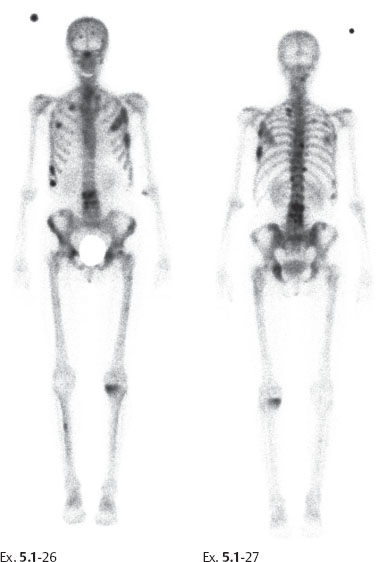
Ex. 5.1-26 & 27 Bone scan. Multiple areas of increased uptake consistent with metastases.
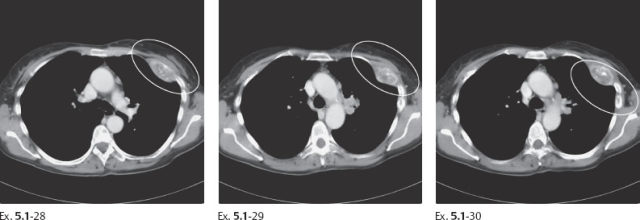
Ex. 5.1-28 to 30 Chest CT, bone window. Destruction of the left anterior third rib.
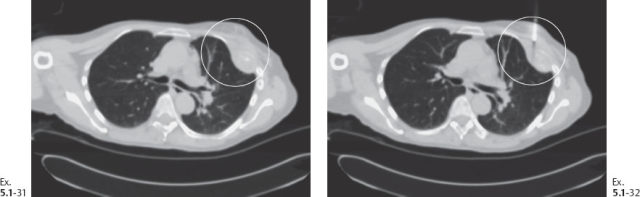
Ex. 5.1-31 & 32 CT-guided large-core needle biopsy of the chest wall metastases.
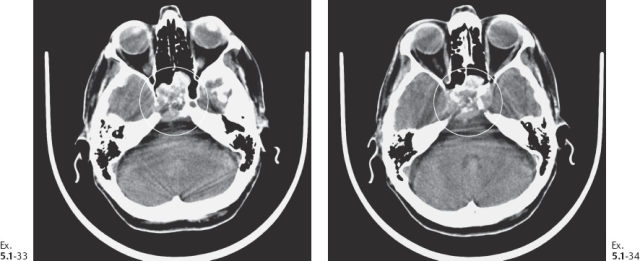
Ex. 5.1-33 & 34 CT showing destruction of the sphenoid sinus.
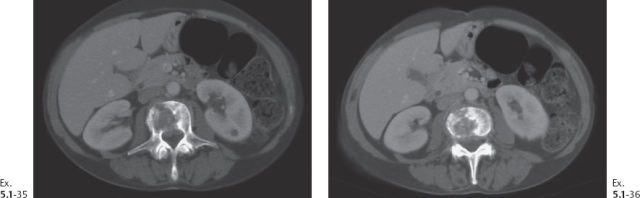
Ex. 5.1-35 & 36 Abdominal CT, bone window. Lumbar vertebral destruction.
Treatment: Sector resection and postoperative irradiation.
Outcome: The patient died 7 years and 1 month following treatment. Autopsy revealed metastatic breast cancer as the cause of death.
Comment
This case clearly demonstrates the disturbing discrepancy between a so-called in situ carcinoma and a fatal outcome, a discrepancy which can be explained by the process of neoductgenesis.
A 51-year-old asymptomatic woman, screening examination.
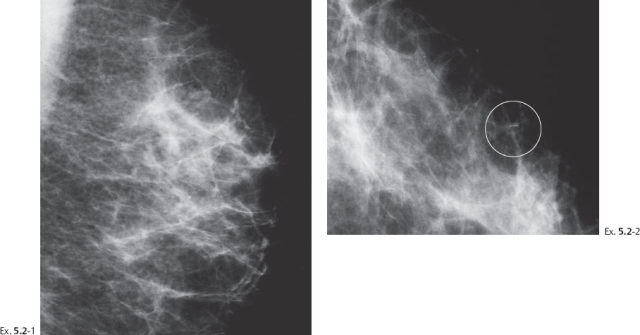
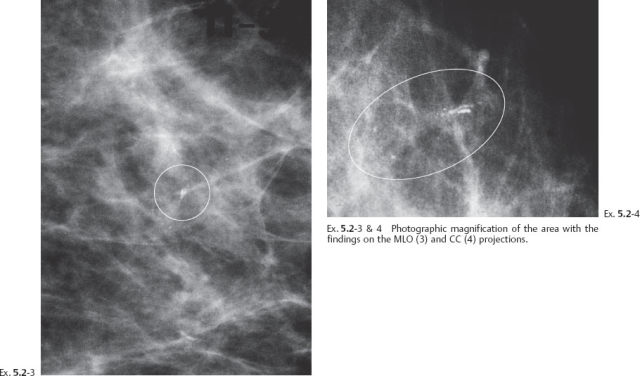

Ex. 5.2-5 Two years after her previous screening examination the patient felt a thickening in the lateral portion of her left breast. Detail of the left CC microfocus magnification view demonstrates innumerable casting type calcifications filling most of the quadrant.
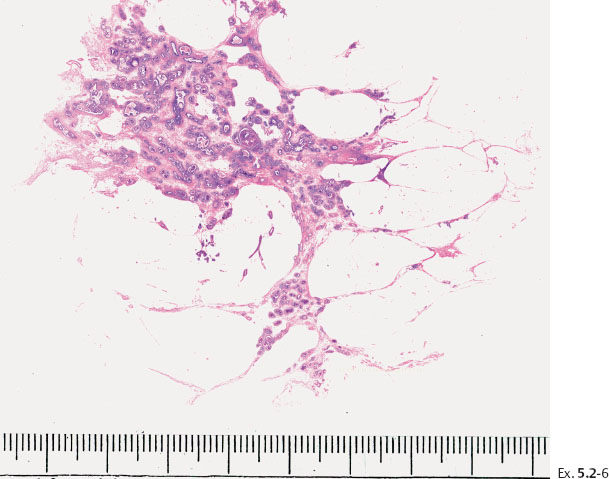
Ex. 5.2-7 Subgross, thick section (3D) histology.
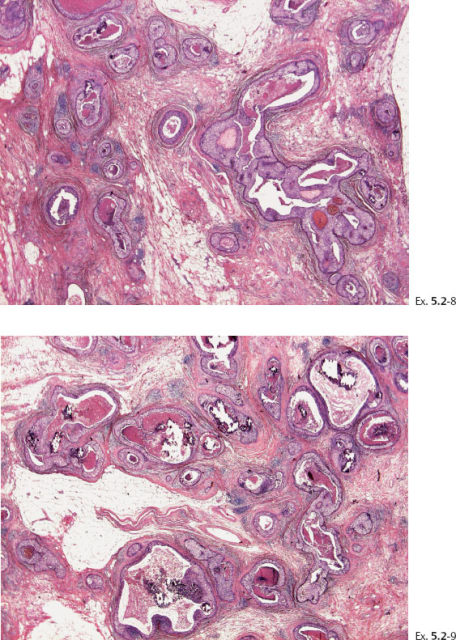
Ex. 5.2-8 & 9 Details of the large-section histology slide.
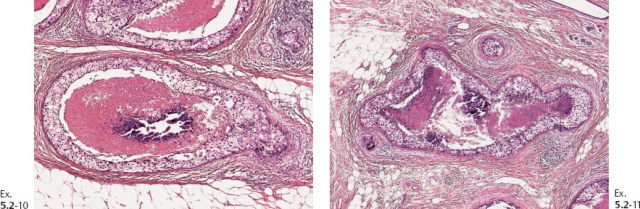
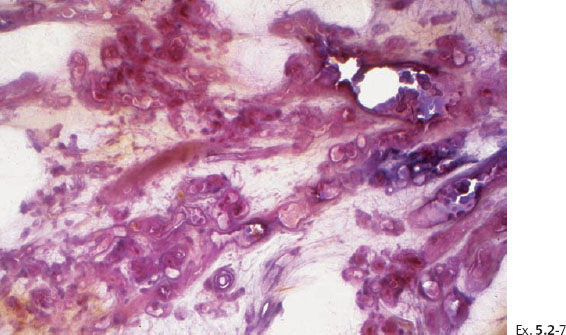
Ex. 5.2-10 & 11 Medium-power histological images (H&E). Ducts in cross section demonstrating solid cell proliferation with extensive central necrosis and amorphous calcification.
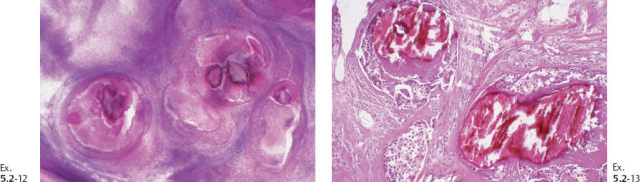
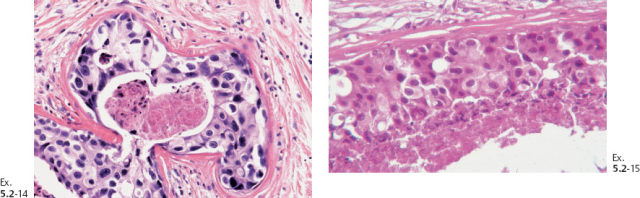
Outcome: The patient died of metastatic breast cancer.
Comment
Again, we find an obvious discrepancy between the so-called in-situ carcinoma and the fatal outcome. Examination of both the large-section subgross (3D) histology and the low-power view of the large thin-section histology specimen reveals the same phenomenon as seen in the previous case, which is an abnormally high concentration of cancerous ducts packed tightly together. These do not resemble preexisting ducts in terms of orientation, size, shape, architecture, desmoplastic reaction or lymphocytic infiltration, or lack of TDLUs, but they are surrounded by a basement membrane.
A 56-year-old asymptomatic woman, screening examination.
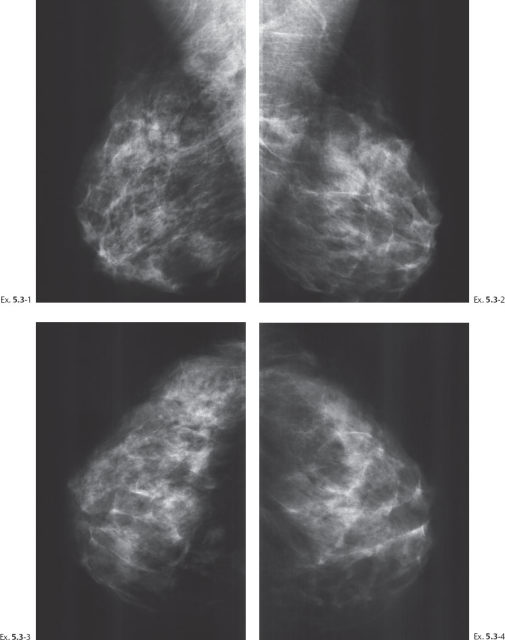
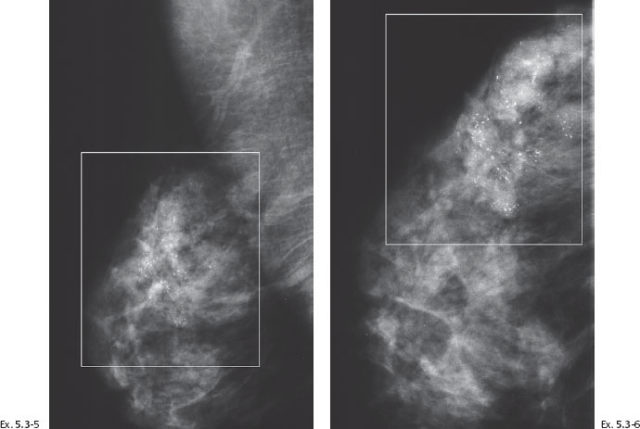
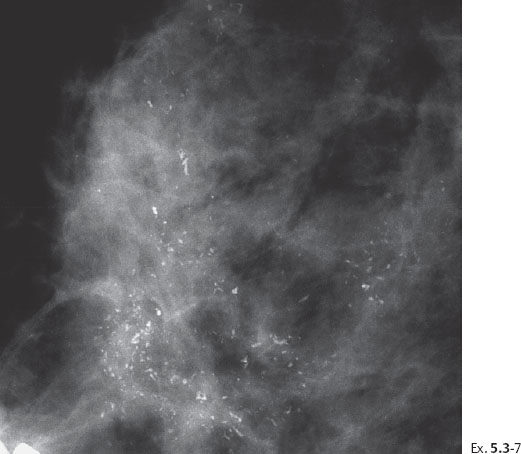
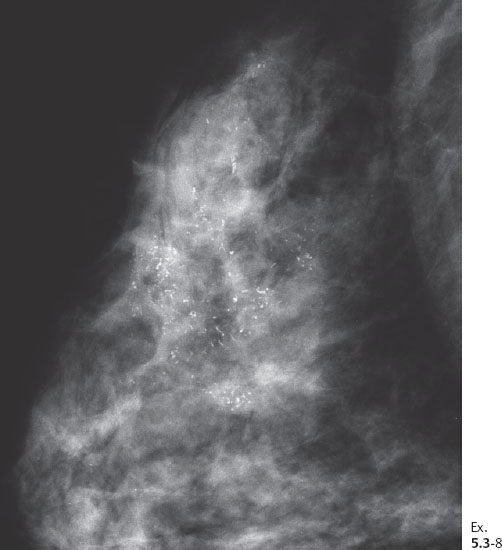
Ex. 5.3-8 Right breast, CC projection, microfocus magnification view, demonstrating the extent and type of the calcifications.
Initial treatment: Sector resection.
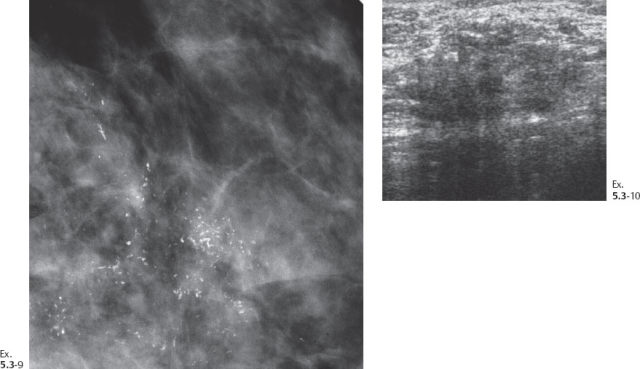
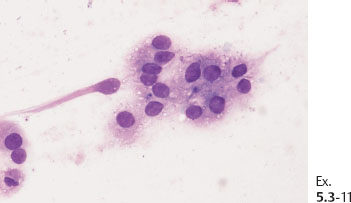
Ex. 5.3-11 Fine-needle aspiration biopsy: malignant cells.
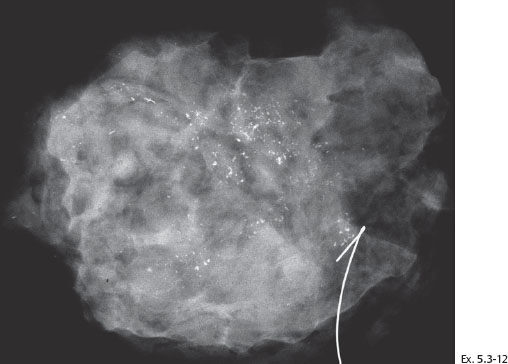
Ex. 5.3-12 Specimen radiograph of the surgical specimen after sector resection shows malignant type calcifications along the resection margin (specimen size 8 cm × 7 cm × 2 cm).
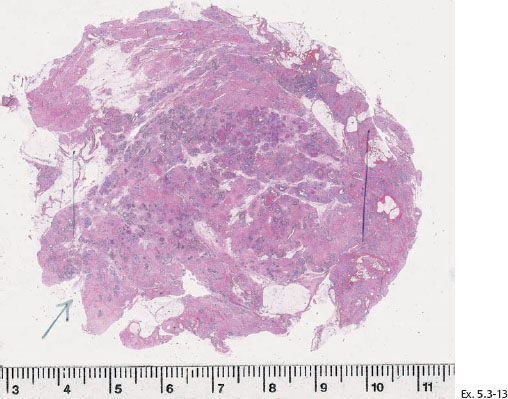
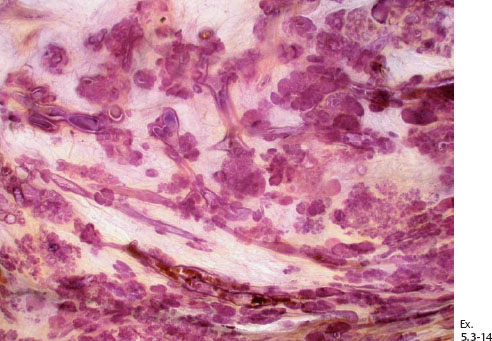
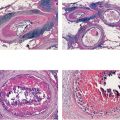
Ex. 5.3-14 Subgross large-section histological image demonstrating numerous cancer-filled, distended ducts in the vicinity of entirely normal glandular structures.
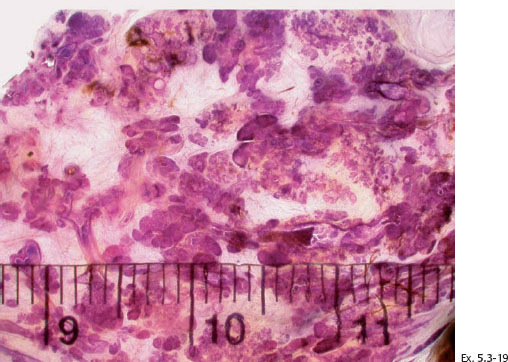
Ex. 5.3-19 Subgross, thick-section (3D) histological image covering several centimeters of this specimen. The cancerous ducts measure more than 1 mm in diameter, approximately 10 times the size of normal ducts.
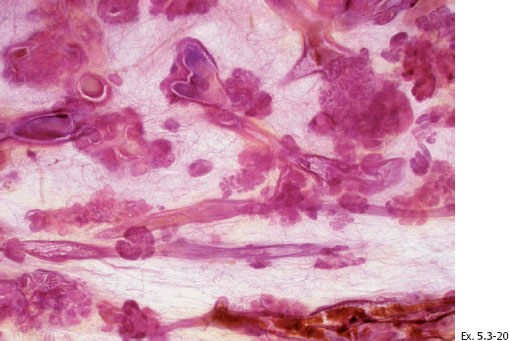
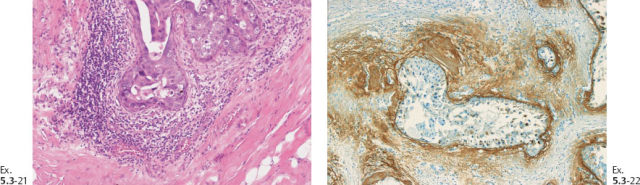
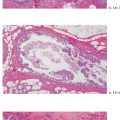
Ex. 5.3-21 & 22 Desmoplastic reaction and lymphocytic infiltration are seen surrounding some of the ducts (21), where tenascin overexpression can also be demonstrated (22).
Further treatment: Subcutaneous mastectomy. Breast reconstruction with a saline implant.
Two years later the patient felt a large lump in her ipsilateral axilla.
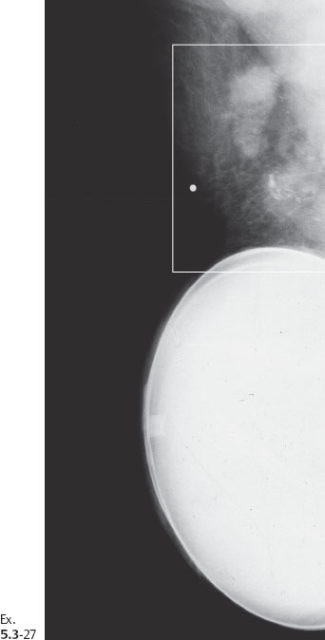

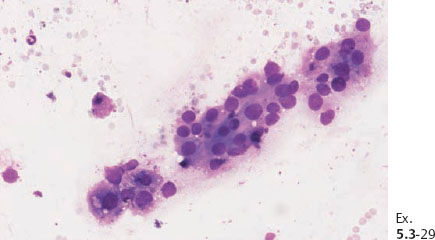
Ex. 5.3-29 Fine-needle aspiration of one of the pathological axillary nodes: malignant cells.
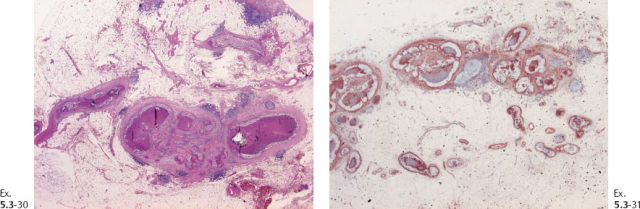
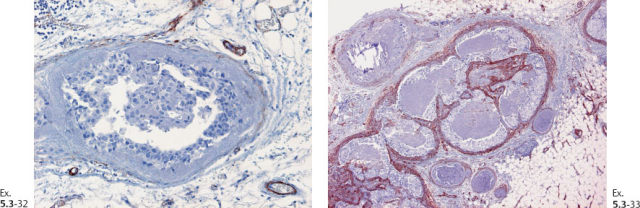
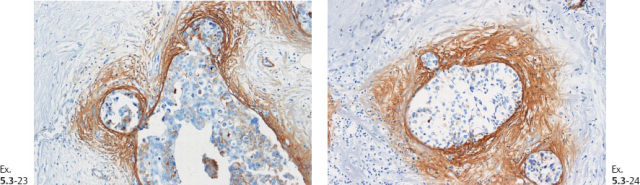
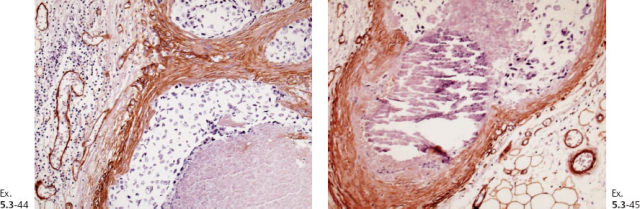
Ex. 5.3-32 & 33 Smooth-muscle staining shows the lack of myoepithelial cell layer around the ductlike structures in the axillary fat. These structures are independent of the lymph nodes and are located in the axillary adipose tissue.
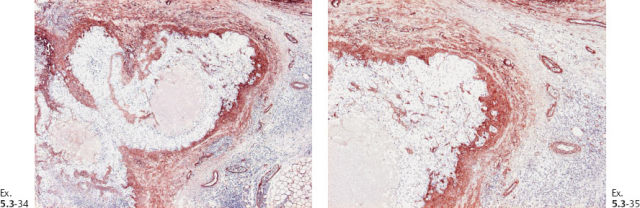
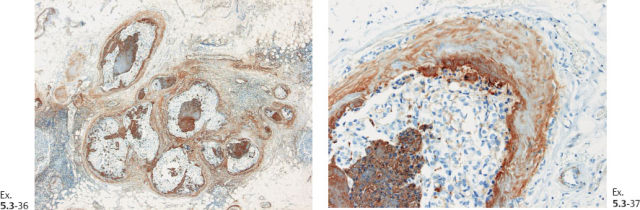
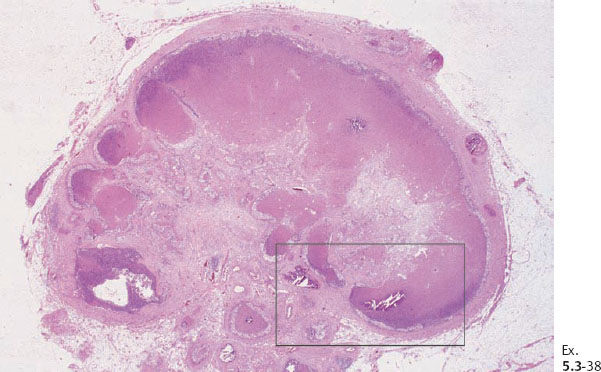
Ex. 5.3-38 Low-power histology image of one of the 12 resected lymph nodes.
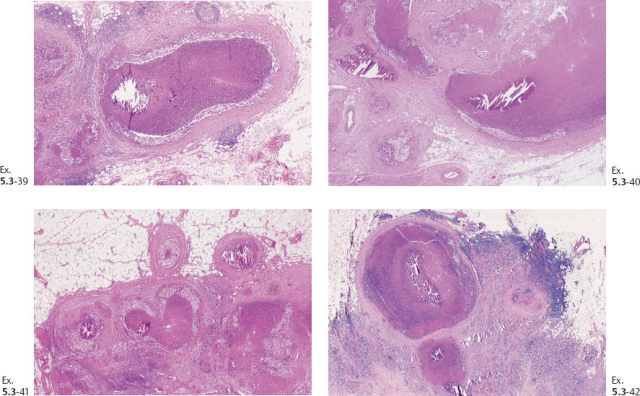
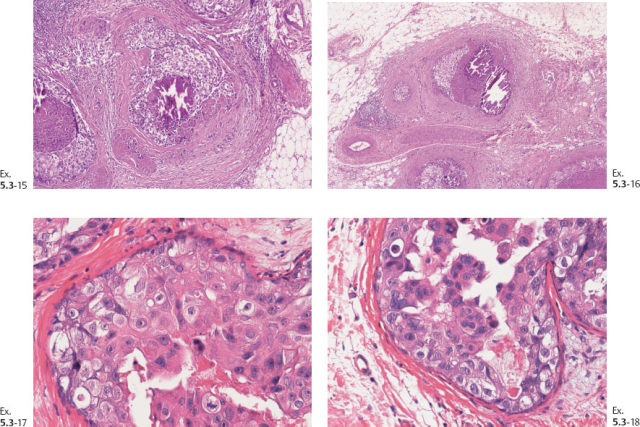
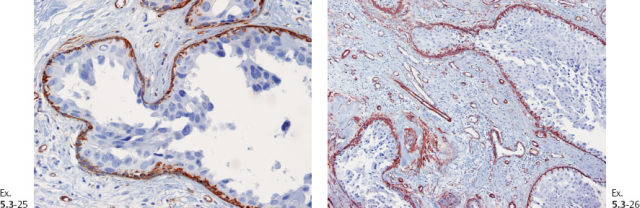
Ex. 5.3-39 to 42 Medium-power histology (H&E) images of one of the axillary lymph nodes show numerous pathological ductlike structures in cross section. These are similar in appearance to that of ductal carcinoma in situ within the breast. The ductlike structures are distended by Grade 3 malignant cells with solid architecture, central necrosis, and amorphous calcifications, and are surrounded by a desmoplastic reaction and lymphocytic infiltration. Ex. 5.3-40 is a magnified image of the area within the rectangle in Ex. 5.3-38.
Adjuvant treatment: Chemotherapy with FEC (fluorouracil [5FU], epirubicin, and cyclophosphamide). Progression of the disease with core biopsy-proven breast cancer metastases to the lungs appearing during treatment. Further disease progression during Herceptin, Taxol and other adjuvant therapy.
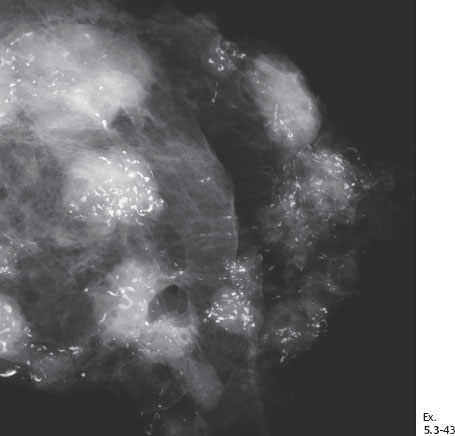
Ex. 5.3-43 Detailed image of the axillary specimen radiograph, showing that both the axillary lymph nodes and the surrounding adipose tissue contain a large number of casting type calcifications.
Outcome: The patient died from breast cancer metastases six years following initial surgery.
Comment
This is an even more complex case than the previous ones, although there are many common features. Similarities include the contradiction of a diagnosis of in-situ carcinoma causing systemic dissemination and the lack of response to adjunctive treatment regimens, including chemotherapy and treatment with Herceptin. There is also the phenomenon of an abnormally high density of cancerous ducts distributed over a large volume of tissue. Again, these ducts lack TDLUs, and their orientation, size, and architecture and the presence of lymphocytic infiltration and desmoplastic reaction distinguish them from normal ducts, although they are still surrounded by a basement membrane.
The differences include the regional metastases to a large number of axillary nodes and even metastases to the axillary adipose tissue. These metastases are strikingly similar in appearance to the “in-situ” component in the breast.