Introduction
Consecutive series of mammograms provide the opportunity to observe the development of breast disease. Concerning the specific breast cancer subtype presenting with casting type calcifications on the mammogram, it appears that one breast lobe might have been genetically malconstructed or damaged during intrauterine life. These genetic alterations of the lobe may remain subclinical for decades. The emergence of this specific breast cancer subtype may show different patterns on the mammogram and on other imaging modalities, as follows:
- No apparent abnormality on the previous mammogram. In some cases the malignant type calcifications seem to appear “suddenly” over a surprisingly large area on the mammogram although the previous examination showed no abnormalities. In these distressing cases the defective lobe with its numerous new branches due to “neoductgenesis” may appear on the mammogram in its entirety within a relatively short period, similarly to a submarine surfacing suddenly from beneath the water (Example on pages 76–79).
- Cluster of calcifications as the earliest sign. In many of the cases, a cluster of crushed stone-like calcifications may be seen on the previous mammogram(s), often associated with one or two linear calcifications, that progress to extensive casting type calcifications on subsequent mammograms (Examples on pages 80–117).
- Subtle casting type calcifications as the earliest sign. In some cases, the earliest mammographically detectable phase of the casting cases may be subtle casting type calcification(s) that have been overlooked (Examples on pages 118–125).
When the initial, subtle sign of this already extensive disease is missed (or the patient is placed on short-term follow-up), the rapid development of this highly malignant process may be manifest on the next mammogram by the appearance of innumerable casting type calcifications over a surprisingly large region of the breast. To pursue the analogy, the conning tower of a submarine will be visible first, while the emergence of the body will follow later.
Magnetic resonance imaging can demonstrate the true extent of the disease earlier and far beyond the mammographically detectable calcifications, because genetic changes predisposing to malignant transformation appear to occur simultaneously throughout much of the lobe.
A Possible Biological Explanation for the Above Observations
The development of the ductal system of the breast is initiated in early embryological life, at which time the number of main ducts is determined. The final arborization of the ducts takes place at puberty. While the number of TDLUs is subject to continuous proliferation and involution during the next few decades, the number of lobes, each with a main duct, will remain constant. If one of the lobes becomes genetically altered during embryological or adult life and acquires a propensity for malignant change, this unique breast cancer subtype can develop. It is characterized by both a malignant transformation of the epithelial cells within preexisting ducts and also by a rapid, disorderly formation of new duct branches. This pattern is in stark contrast to that of other breast cancer subtypes, which are characterized by malignant transformation of the TDLUs involving only part of a lobe. The genetic changes predisposing to malignancy and the subsequent malignant process are both able to involve the duct system of an entire lobe (whatever its size). The result is a complex conglomerate of both preexisting and newly formed neoplastic ducts. This suggested process may explain the seemingly sudden emergence of extensive disease on the mammogram, although functional imaging methods, such as contrast-enhanced MRI of the breast, may detect the presence of disease considerably earlier and over a larger extent than does the mammogram.
In any case, the diagnostic and therapeutic team members need to be aware of the frequently extensive and highly fatal nature of this special subtype of breast cancer.1–9
No Apparent Abnormality on the Previous Mammogram
Only one case (Ex. 2.1) is presented here. The first group comprises cases in which no mammographic sign of the presence of the disease was present at the time of the previous examination.
Example 2.1
A 69-year-old asymptomatic woman, screening examination.
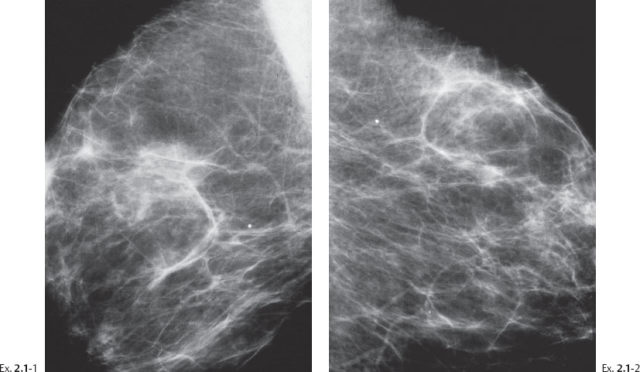
Ex. 2.1-1 & 2 Right and left breasts, MLO projections. No mammographic abnormality is seen.
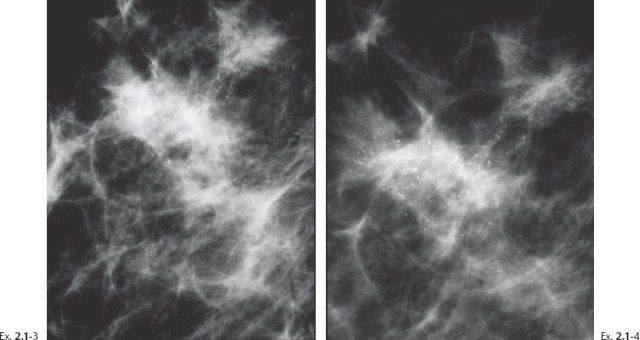

Ex. 2.1-5 Right breast, detail of the MLO projection, microfocus magnification.
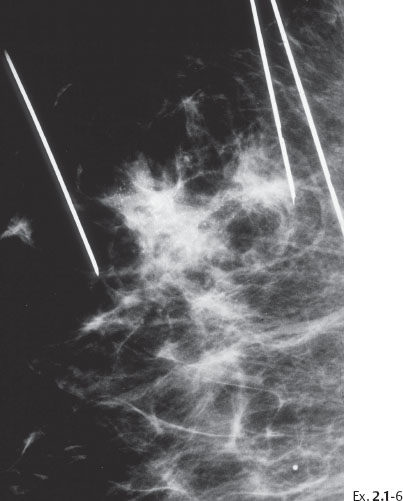
Ex. 2.1-6 Preoperative localization: bracketing the pathological lesions using multiple wires.
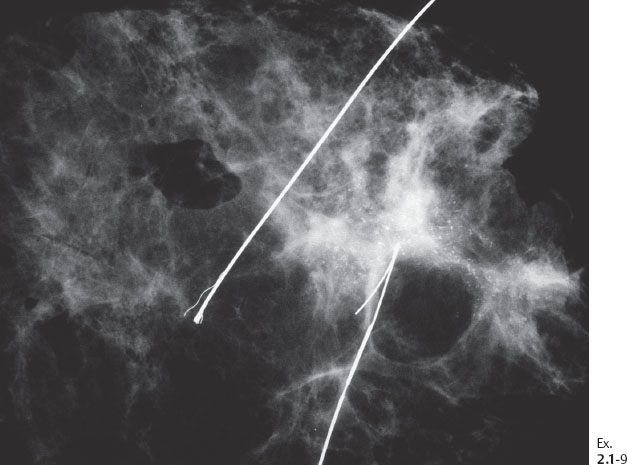
Ex. 2.1-9 Specimen radiograph demonstrating the calcifications.
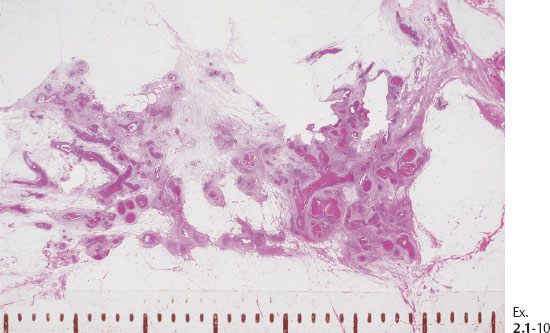
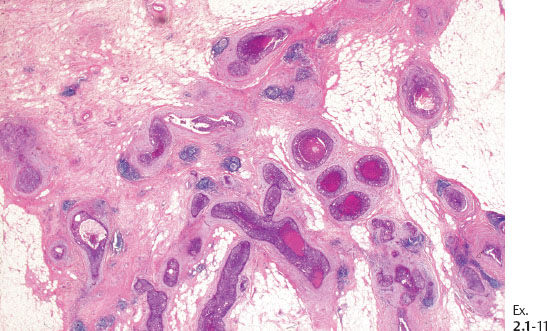
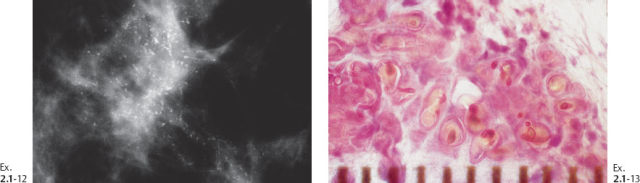
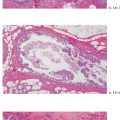
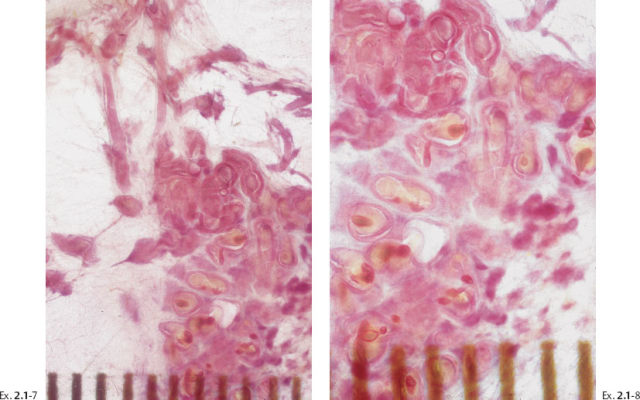
Ex. 2.1-12 & 13 Comparative mammographic and thick-section histological images of this rapidly developing Grade 3 “in situ” process. The millimeter scale shows that the individual ducts are extremely distended by the pathological process.
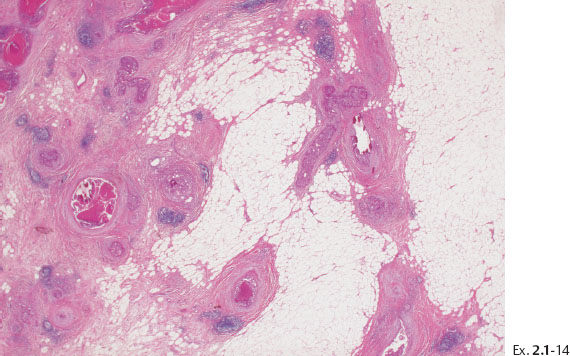
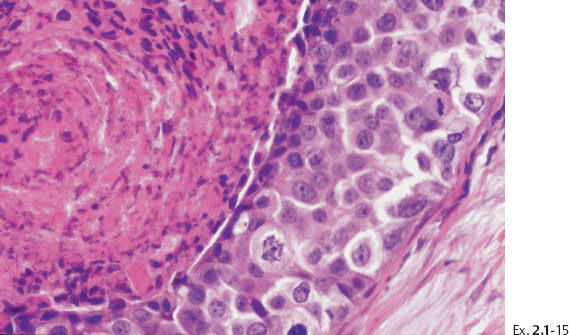
Treatment and follow-up: Mastectomy. The patient was recurrence-free at the most recent follow-up examination seven years after surgery.
Cluster of Calcifications as the Earliest Sign
The second group comprises cases in which very subtle early signs of the presence of the disease have not been fully appreciated. The crushed stone-like calcifications may represent a precursor of castings. We can speculate from the underlying histology that if the malignant process producing crushed stone-like calcifications is not removed surgically, it could progress to widespread disease producing casting type calcifications. Judging from the underlying histology, these would have developed to casting type calcifications over a large area had they not been removed surgically.
Example 2.2
A 43-year-old asymptomatic woman, screening examination. A single cluster of calcifications was not perceived at screening.
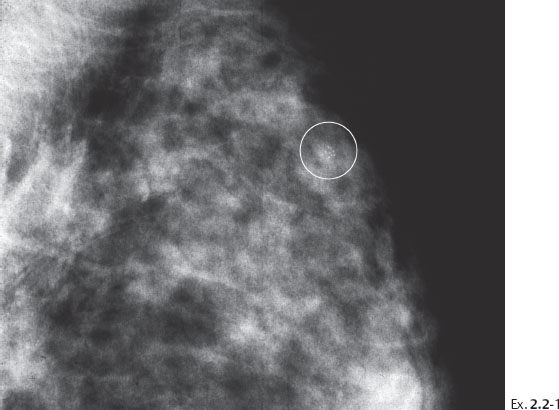
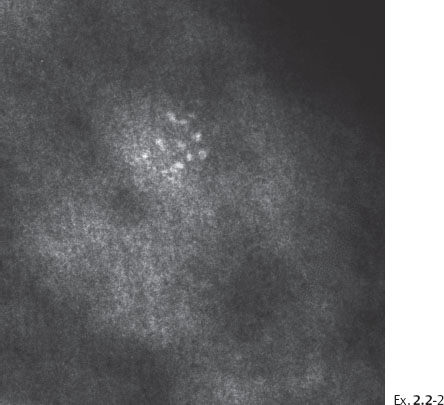
Ex. 2.2-2 Photographic magnification of the cluster of crushed stone-like calcifications.
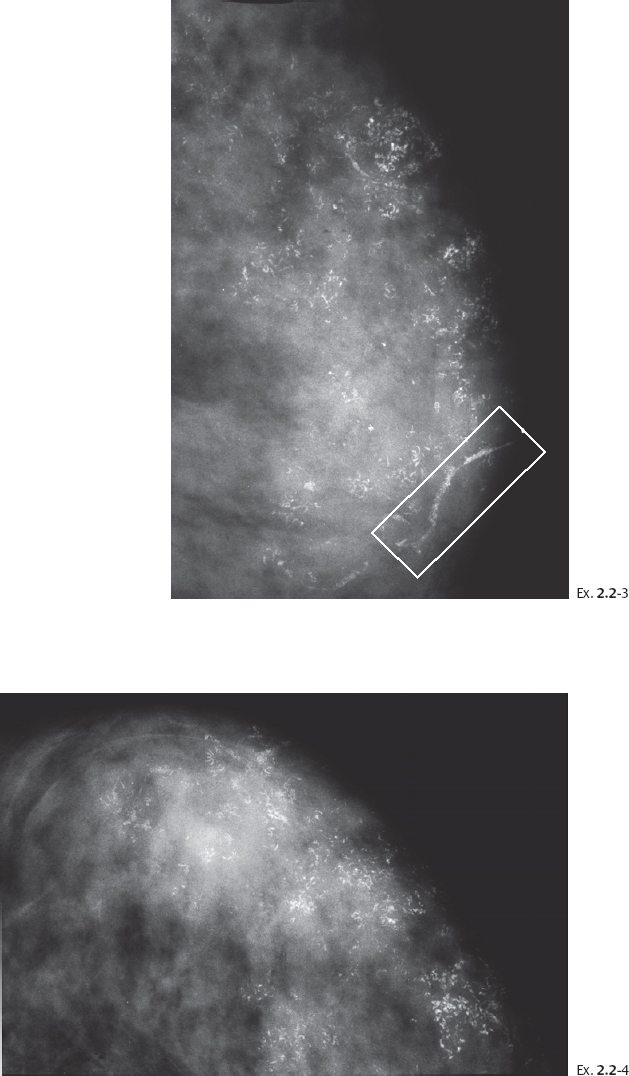
Ex. 2.2-3 & 4 Twenty-five months later, still asymptomatic, screening examination. Left MLO (3) and CC (4) projections. Innumerable casting type calcifications have appeared since the previous examination, and are spread over two-thirds of the breast. No associated tumor mass is visible.
Although the first visible calcification cluster was localized in the upper outer quadrant, the calcifications are now found not only in the upper half of the breast but also fill part of the lower half of the breast, with the corresponding main duct being also filled with malignant type calcifications (rectangle).
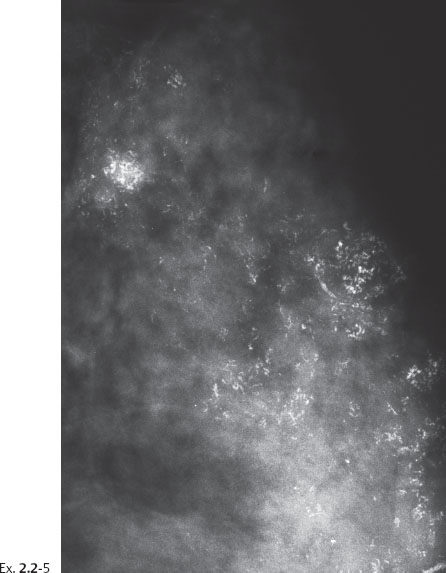
Ex. 2.2-5 Detail of the lateromedial horizontal projection.
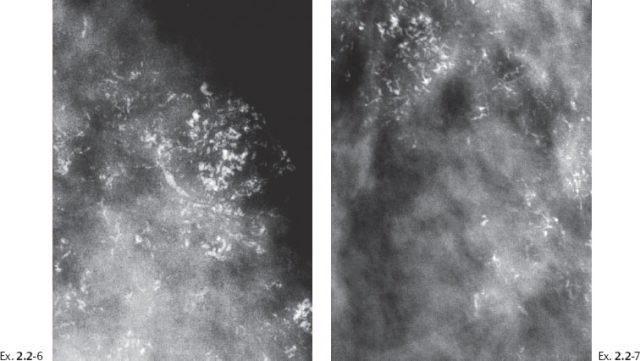
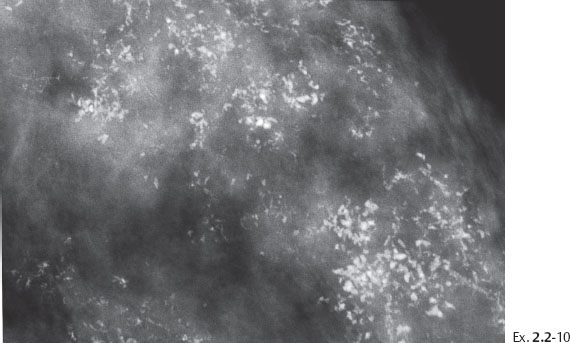
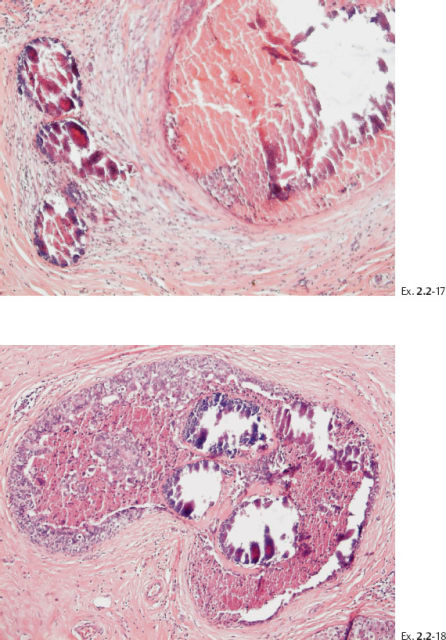
Ex. 2.2-10 The calcifications demonstrate a plethora of ducts containing both types of casting type calcifications.
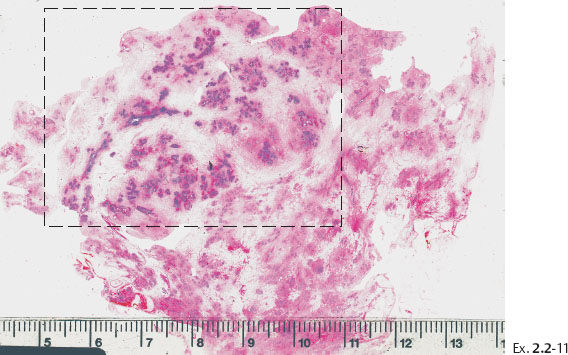
Ex. 2.2-11 Large-section histological image. The cancerous ducts occupy a large area (rectangle).
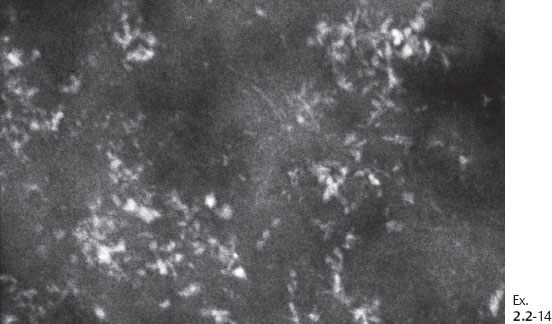
Ex. 2.2-14 Additional magnification image of this case.
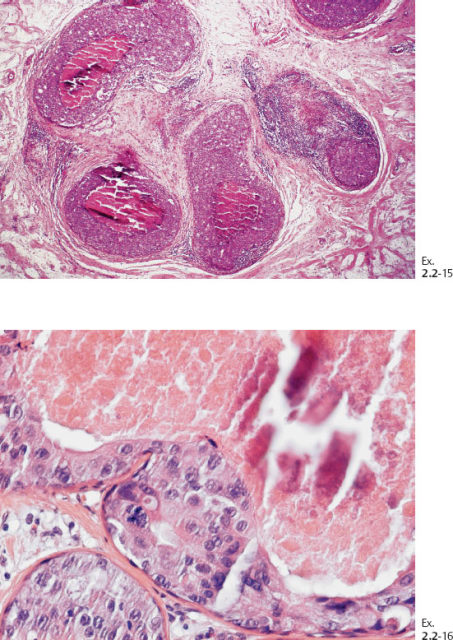
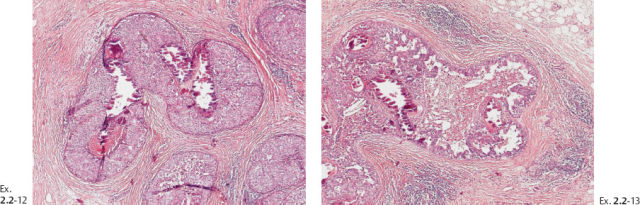
Ex. 2.2-17 & 18 Histological slides demonstrating the localization of some of the dotted casting type calcifications.
Treatment and follow-up: mastectomy without adjunctive treatment. The patient was recurrence-free at the most recent follow-up examination 16 years after surgery.
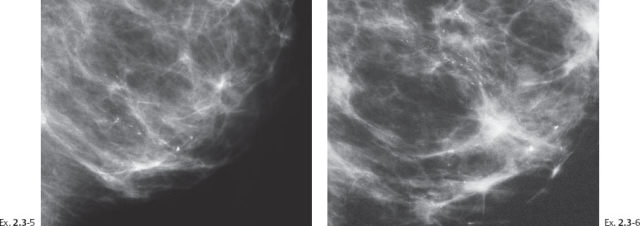
A 51-year-old asymptomatic woman, screening examination.
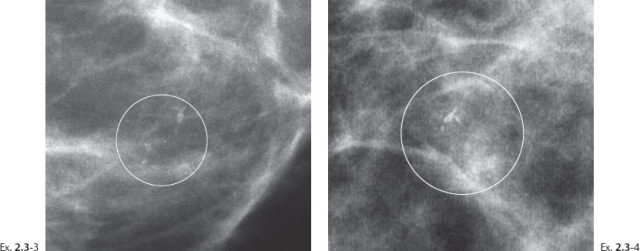
Ex. 2.3-3 & 4 Photographic magnification of the area with the calcifications.
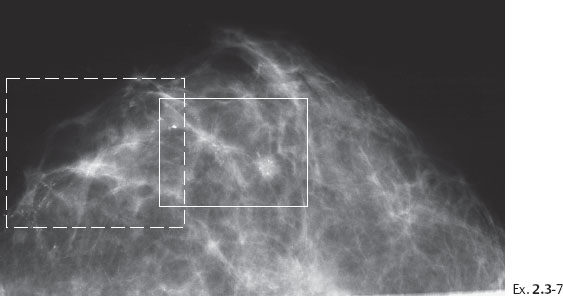
Ex. 2.3-7 CC projection demonstrating the extensive calcifications and a tiny stellate lesion.
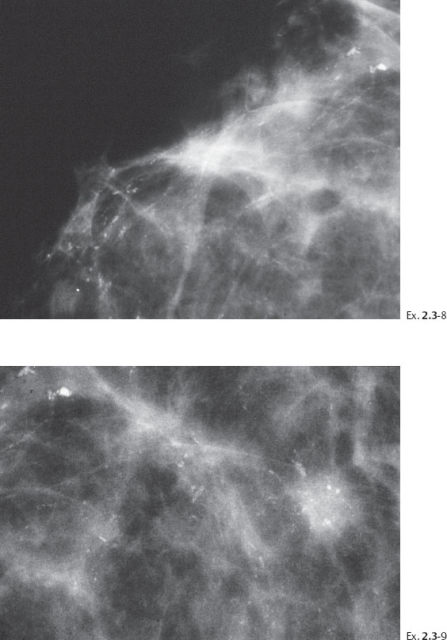
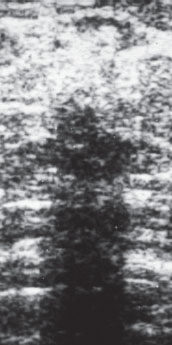
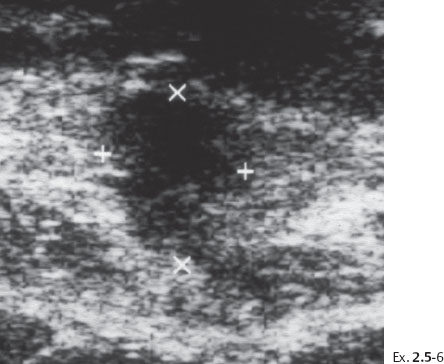
Ex. 2.3-10 Ultrasound image of the stellate lesion.
Ex. 2.3-11 Specimen radiograph of an ultrasound guided 14G core biopsy sample.
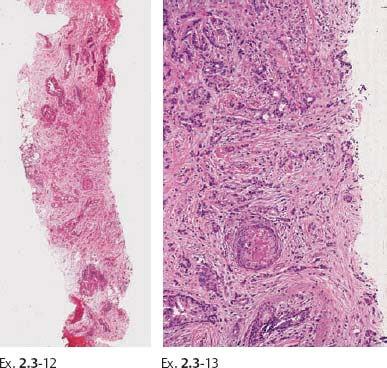
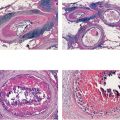
Ex. 2.3-12 & 13 Histology of the core sample: Grade 2 invasive ductal carcinoma.
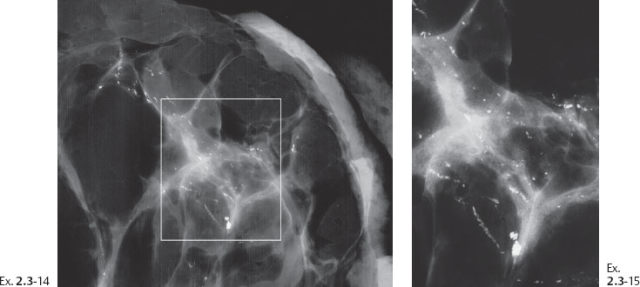
Ex. 2.3-14 & 15 Microfocus magnification radiographs of 5-mm thick specimen slices.
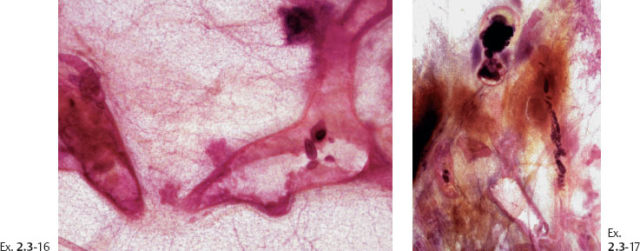
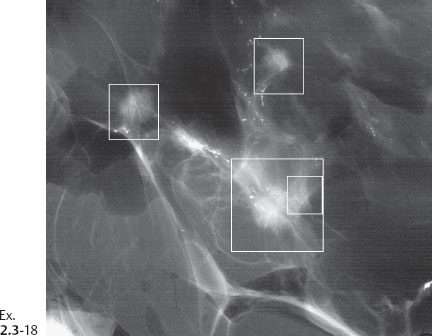
Ex. 2.3-18 One of the specimen slices reveals four tiny stellate lesions.
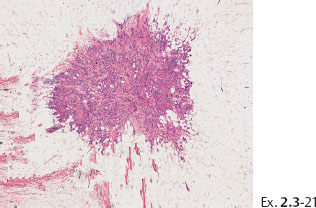
Ex. 2.3-21 The largest of the four invasive cancer foci (3 mm × 3 mm).
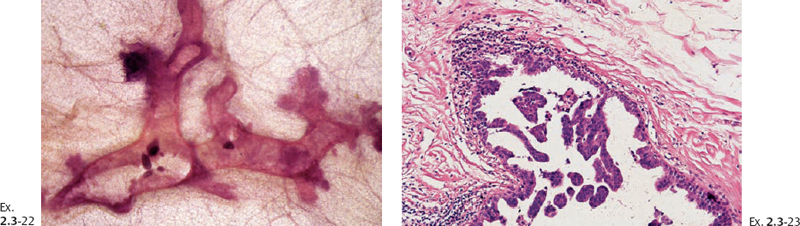
Treatment and follow-up: Mastectomy. No recurrence was demonstrable at her most recent follow-up at 9 years and 6 months after treatment.
A 58-year-old woman presented with a palpable, hard lump in the upper outer quadrant of her right breast. Her previous screening mammogram taken 16 months earlier had been interpreted as normal.
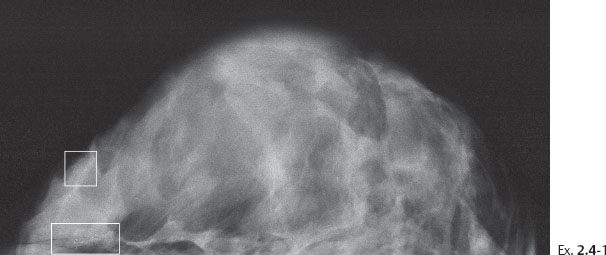
Ex. 2.4-1 Right breast, CC projection, previous screening examination at age 56 years.
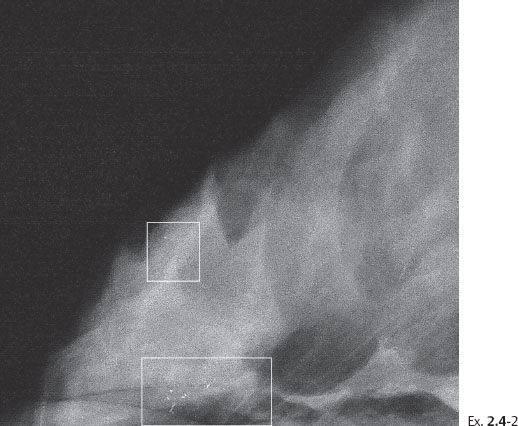
Ex. 2.4-2 Photographic magnification of the regions outlined in Ex. 2.4-1. The upper rectangle shows one nonspecific calcification; the lower rectangle outlines a small cluster of calcifications without an associated tumor mass.
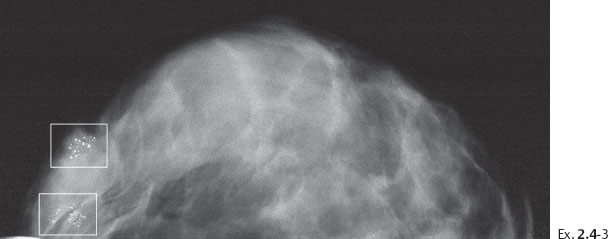
Ex. 2.4-3 Right breast, CC projection, 16 months after the previous mammographic examination, now aged 58.
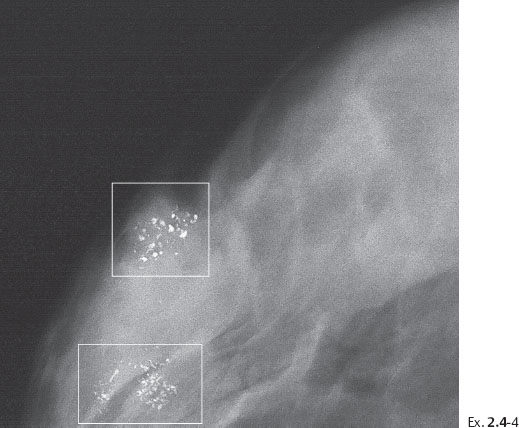
Ex. 2.4-4 Photographic magnification of the regions outlined in Ex. 2.4-3. The upper rectangle shows predominantly crushed stone-like calcifications, while in the lower rectangle there is a mixture of crushed stone-like and casting type calcifications.
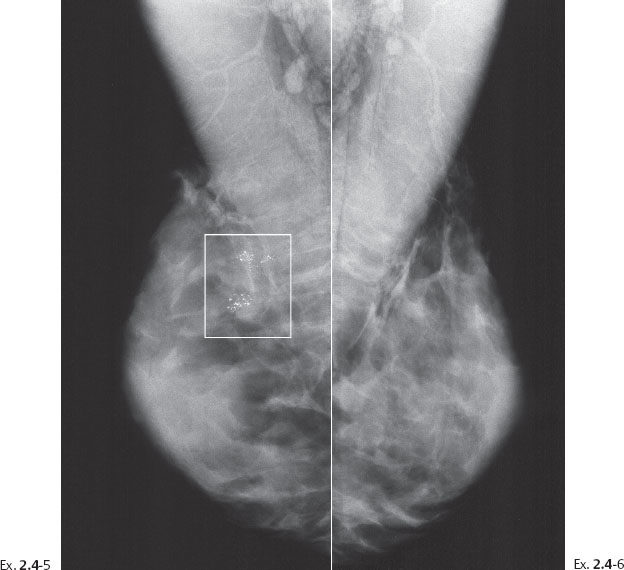
Ex. 2.4-5 & 6 Right and left breasts, MLO projections. Multiple clusters of calcifications are seen outlined by the rectangle.
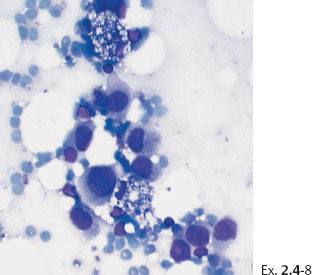
Ex. 2.4-8 Fine-needle aspiration biopsy of the palpable tumor: malignant cells.
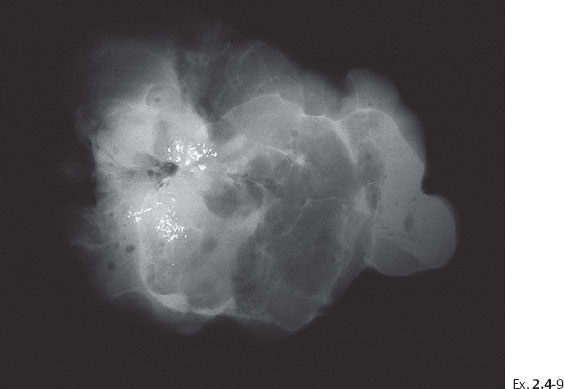
Ex. 2.4-9 Operative specimen radiograph with multiple clusters of calcifications.
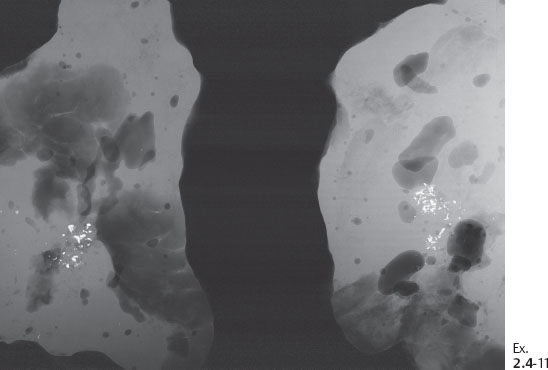
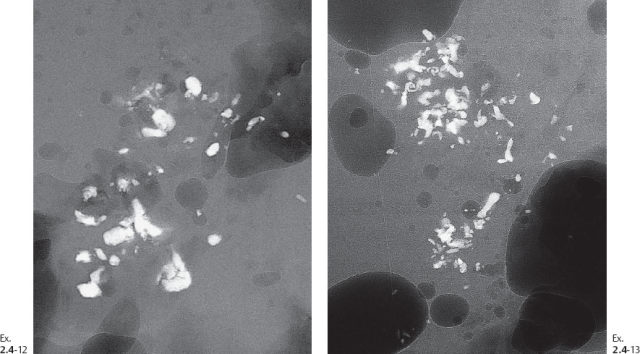
Ex. 2.4-11 Radiographs of the specimen slices demonstrating the clusters of calcifications.
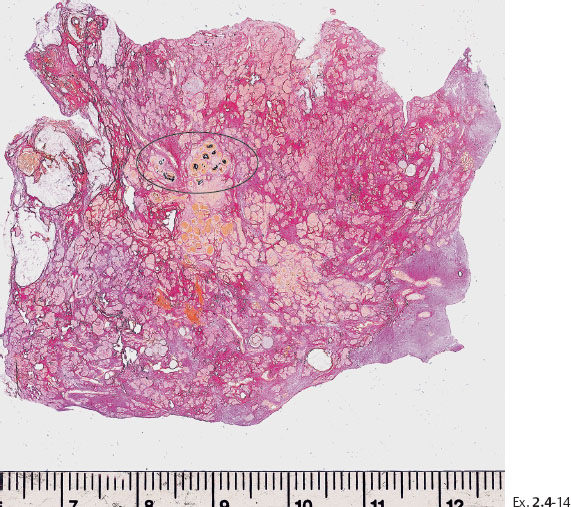
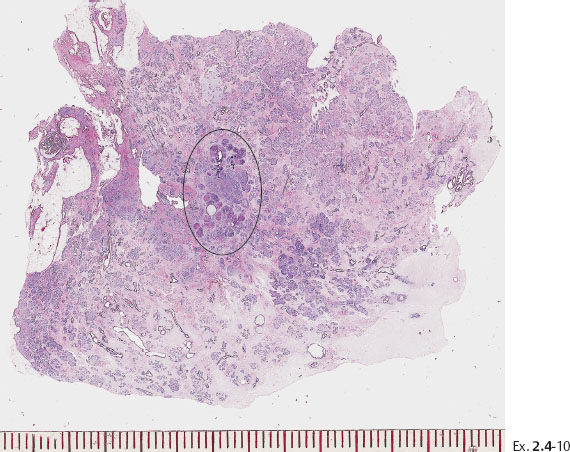
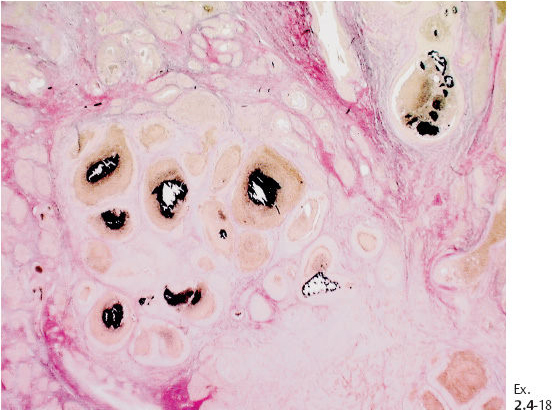
Ex. 2.4-14 Large-section histological image of a slice containing some of the calcifications and surrounding tissue. Combined H&E and von Kossa staining. The clusters of calcifications, stained in black, are encircled.
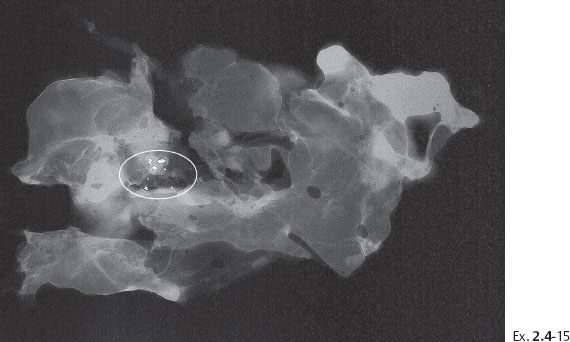
Ex. 2.4-15 Radiograph of one of the specimen slices with a cluster of calcifications.
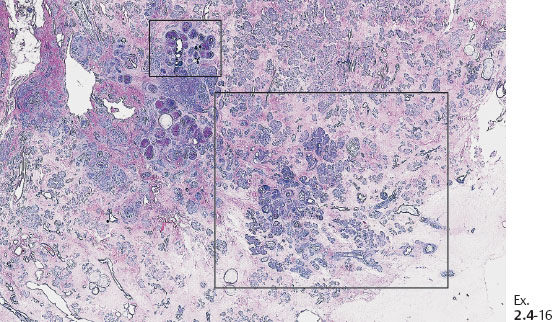
Ex. 2.4-16 Large-section histological image (H&E stain) of a slice containing the calcifications. The smaller rectangle outlines the area with calcified DCIS; the larger rectangle shows the area containing noncalcified DCIS, measuring together 50 mm × 30 mm.
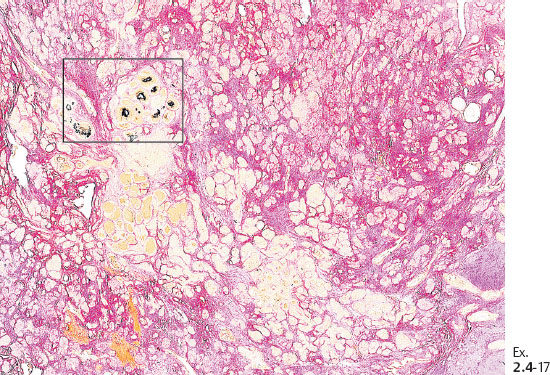
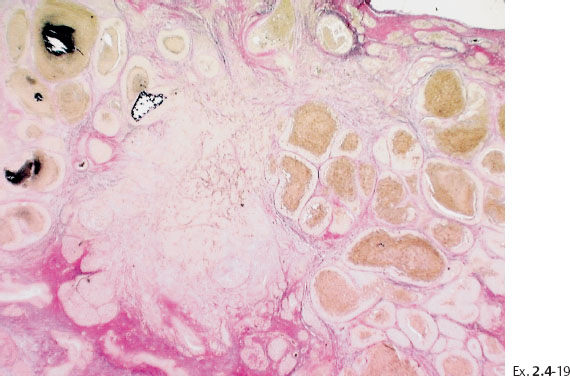
Ex. 2.4-19 Calcified and noncalcified DCIS foci side by side.
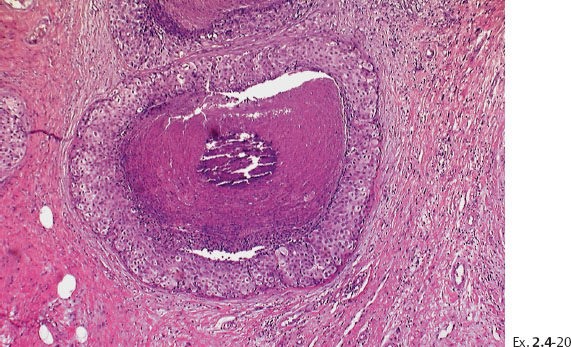
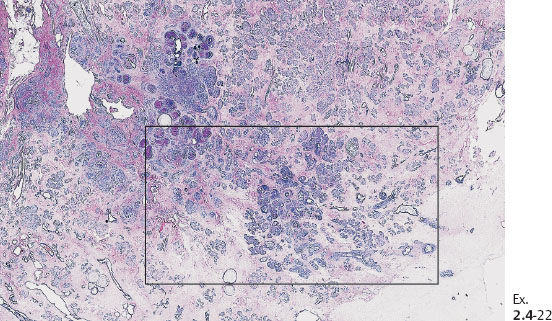
Ex. 2.4-22 Large-section histology (H&E stain). The rectangle outlines the area with noncalcified DCIS.
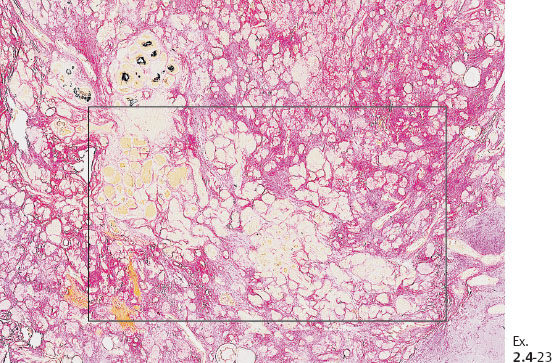
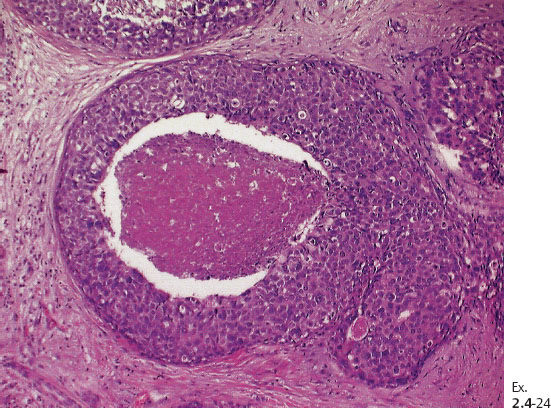
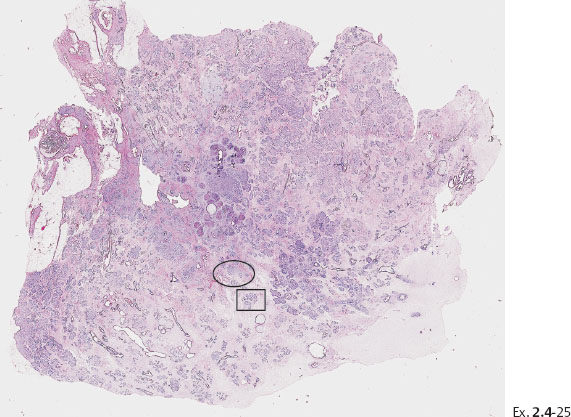
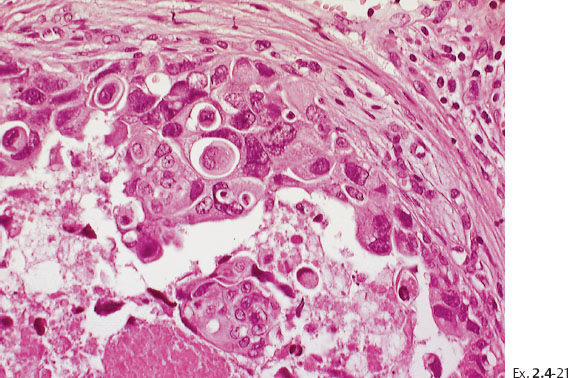
Ex. 2.4-25 Large-section histological image (H&E stain). The part of the image outlined by the oval mark contains a tiny focus of Grade 2 invasive ductal carcinoma (detail image in Ex. 2.4-26). An additional 8 mm focus was found in another histologic section. The rectangle outlines lymph vessel invasion (see Ex. 2.4-27).
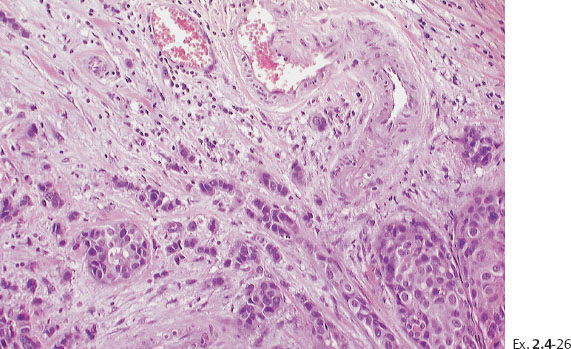
Ex. 2.4-26 Higher-magnification of the area within the oval-shaped mark in Ex. 2.4-25, showing a 2 mm Grade 2 invasive ductal carcinoma.
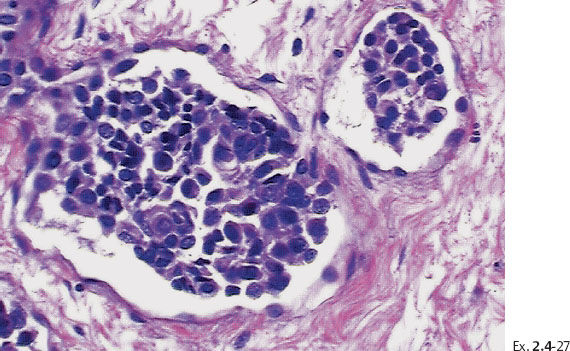
Treatment and follow-up: Mastectomy. The patient was free from recurrence at the most recent follow-up, four years after treatment.
A 55-year-old asymptomatic woman, screening examination.
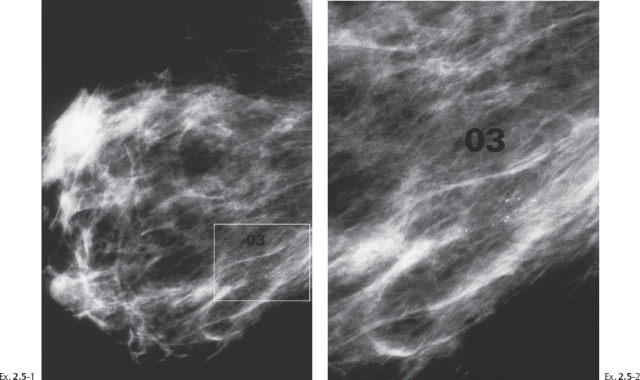
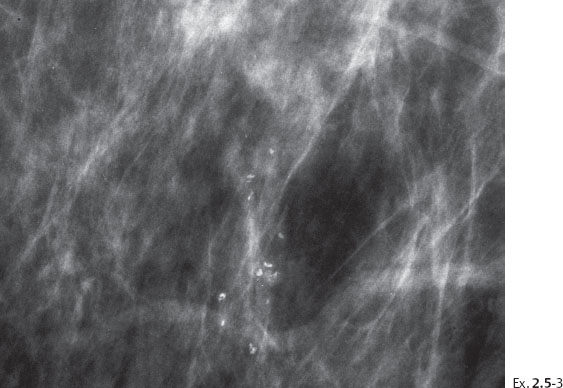
The patient returned after six months with an obvious, palpable tumor.
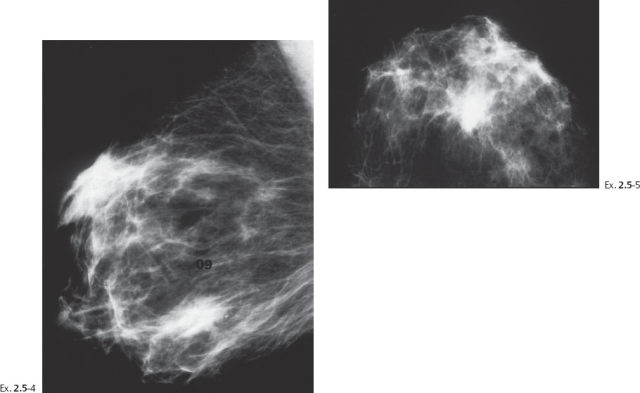
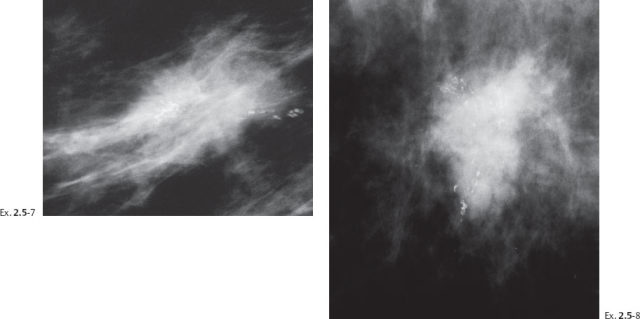
Treatment and follow-up: Mastectomy. The patient moved and was lost to follow-up.
Screening examination of a 64-year-old asymptomatic woman.
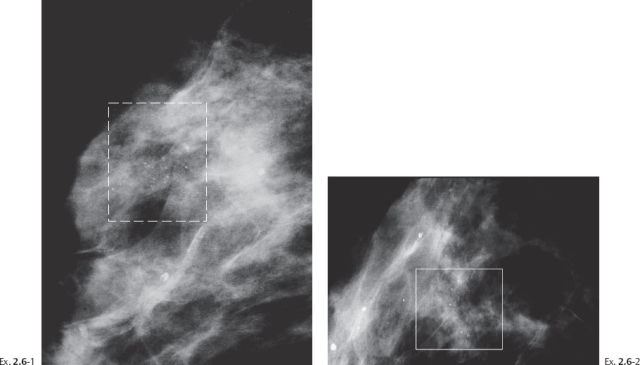
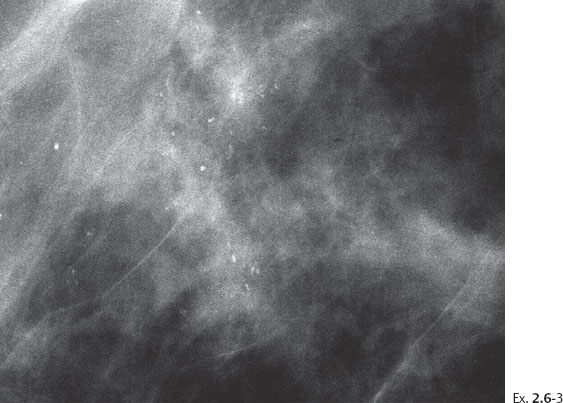

Ex. 2.6-4 Screening examination two years later, still asymptomatic. Right breast, detail of the MLO projection. The calcifications now occupy a larger area.
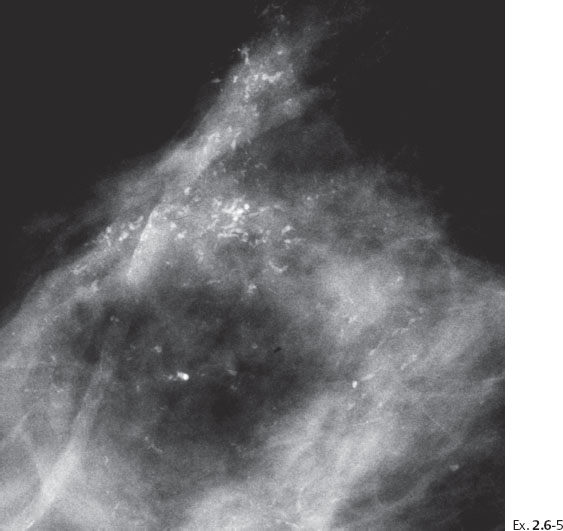

Ex. 2.6-6 Right breast, CC projection.
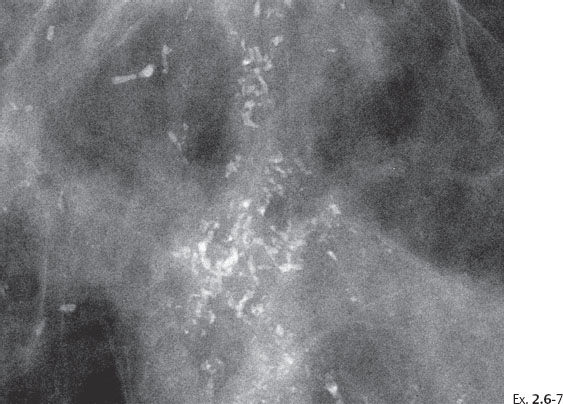
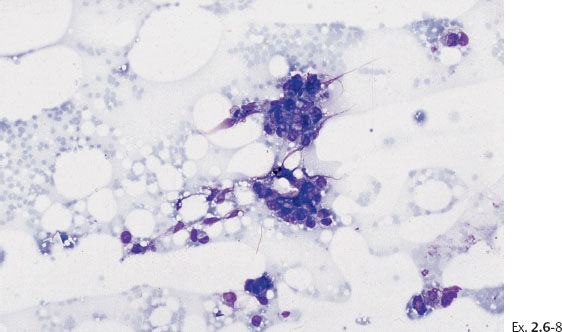
Ex. 2.6-8 Fine-needle aspiration biopsy: atypical cells.
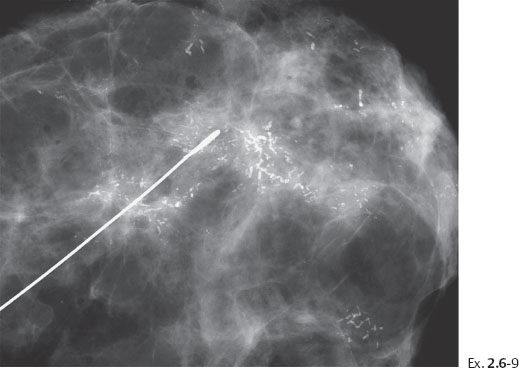
Ex. 2.6-9 Specimen radiograph. The calcifications are seen on the surgical margin.
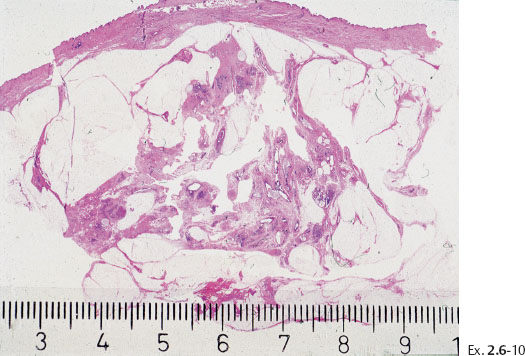
Ex. 2.6-10 Large, thin-section (3–4 μm) histology: 55 mm Grade 3 DCIS.

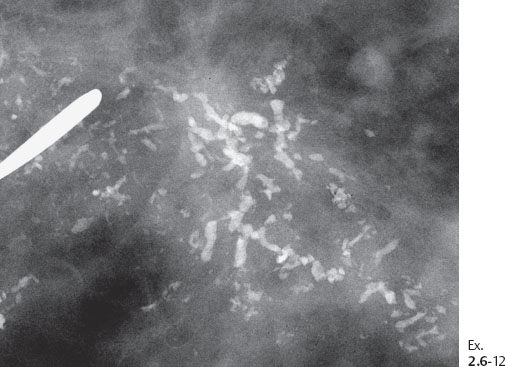
Ex. 2.6-12 Detail of a microfocus specimen radiograph.
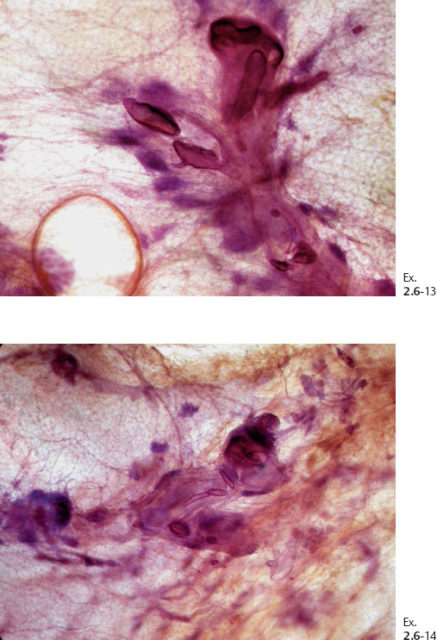
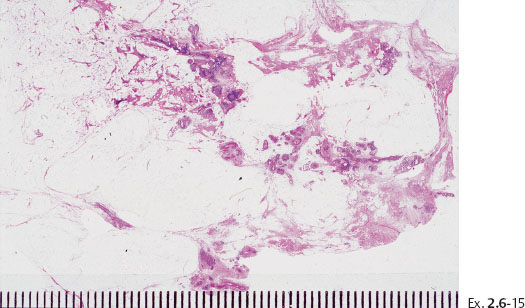
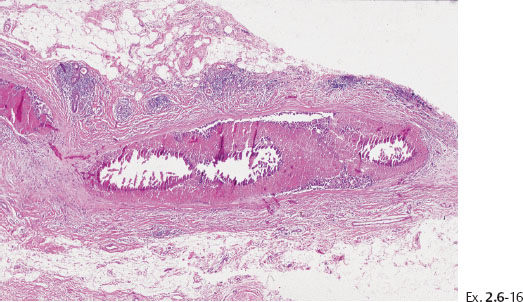
Ex. 2.6-15 Large, thin-section histology showing the extent of the disease.
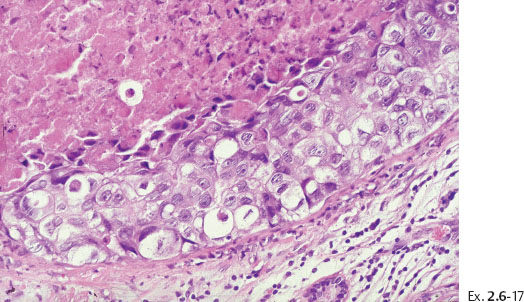
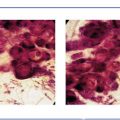
Ex. 2.6-17 High-power magnification showing the Grade 3 cancer cells.
Treatment and follow-up: Mastectomy. The histological examination showed an additional 40 mm focus of DCIS. The patient was symptom-free at the most recent follow-up examination seven years after treatment.
(Case courtesy of Dr. Angela Sie, M.D., Long Beach, CA, USA) A 52-year-old asymptomatic woman, screening examination. A single cluster of calcifications was detected at screening.
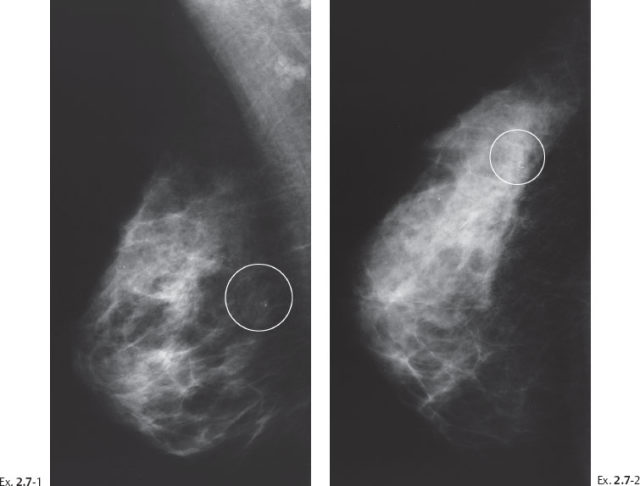
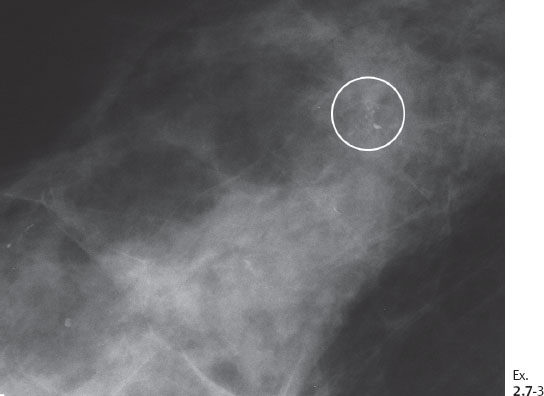
Ex. 2.7-3 Microfocus magnification view: Mammographically malignant type calcifications.
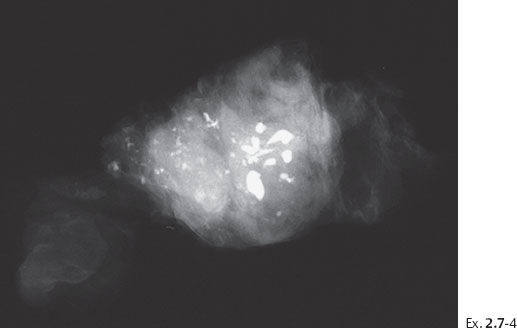
Ex. 2.7-4 Preoperative large-bore needle biopsy showing a representative sample of calcifications contained in the specimen. Histology: Grade 2 & 3 DCIS.
Treatment and follow-up: Mastectomy. The histological examination showed Grade 2 and Grade 3 DCIS with multiple foci of Grade 2 invasive cancers (< 10 mm). The patient was symptom-free at the most recent follow-up examination two years after treatment.
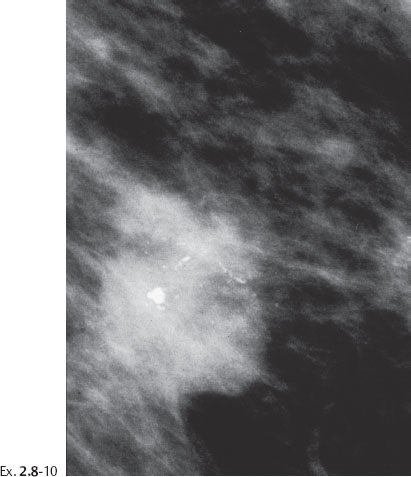
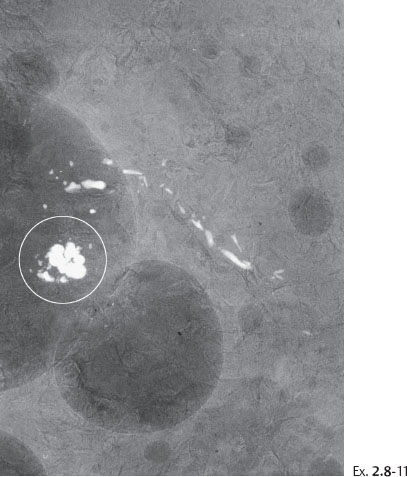
A 53-year-old asymptomatic woman, screening examination.
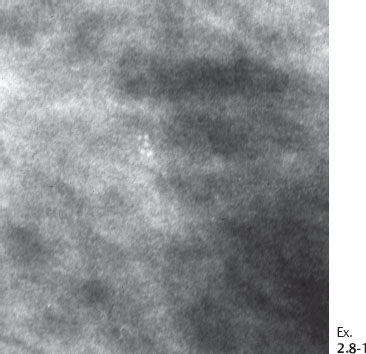
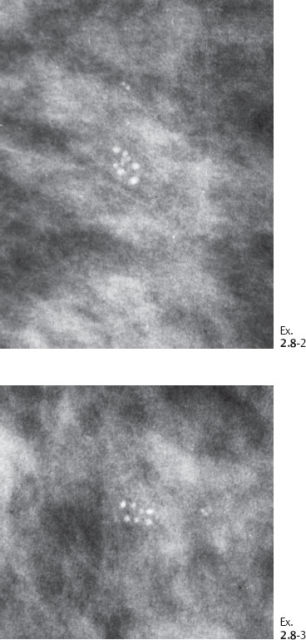
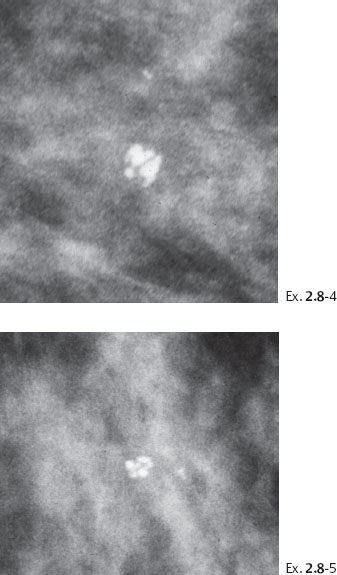
Ex. 2.8-4 & 5 An additional two years later the patient was still asymptomatic. The individual calcifications have become larger and coarser. No associated tumor mass is seen.
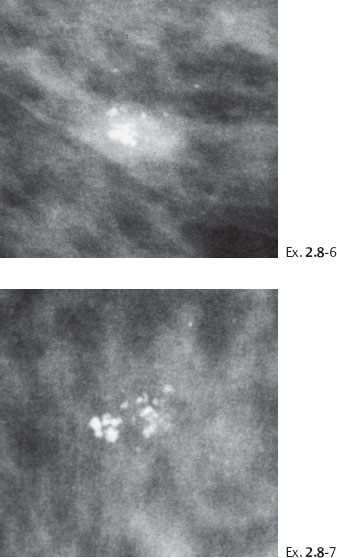
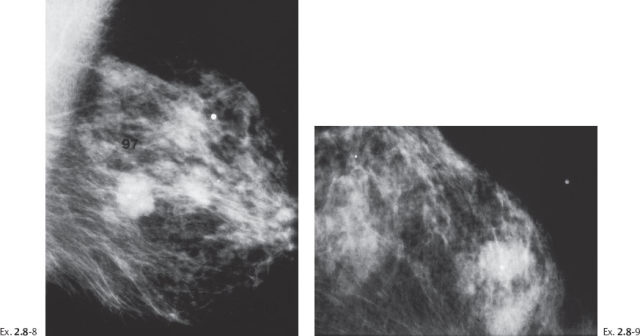
Ex. 2.8-8 & 9 Two years after her last screening examination the patient felt a lump in the upper outer quadrant of her left breast. Detail of the MLO and CC projections. There has been a dramatic change during the past two years, in contrast to the gradual changes over the previous seven years.
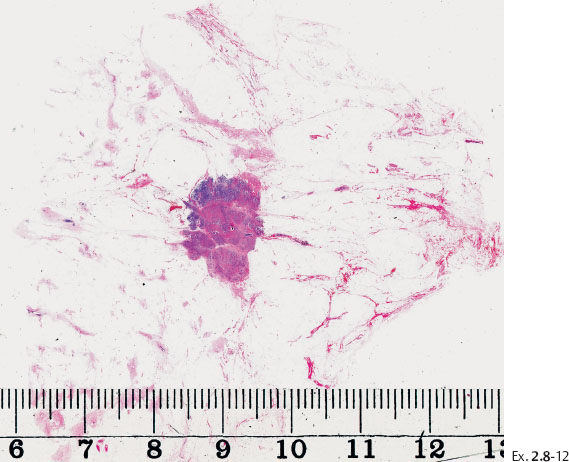
Ex. 2.8-12 Large-section histological image of the tumor and its immediate surroundings (H&E).
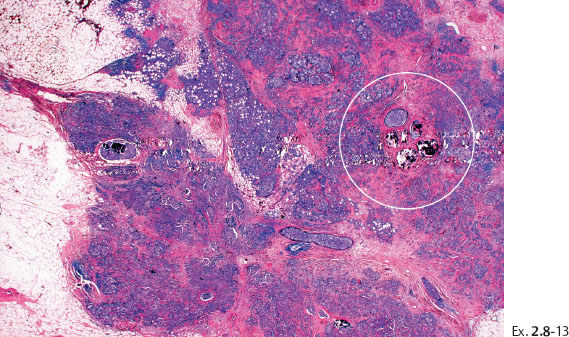
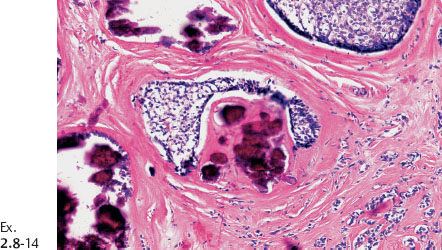
Ex. 2.8-14 High-power histological image (H&E).
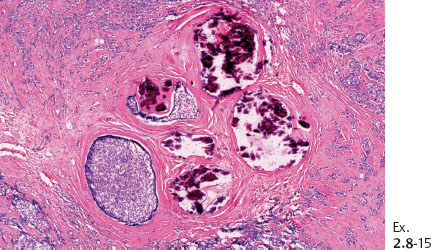
Ex. 2.8-15 The calcifications are surrounded by invasive and in situ carcinoma (H&E).
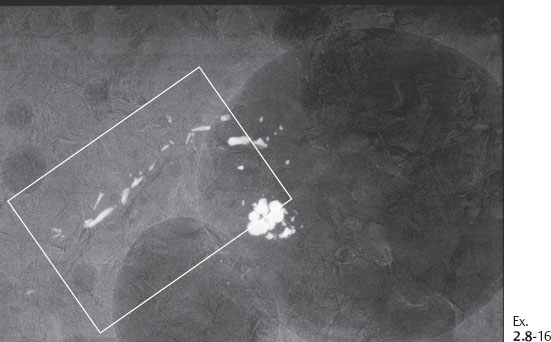
Ex. 2.8-16 Specimen radiograph of the paraffin block containing the calcifications.
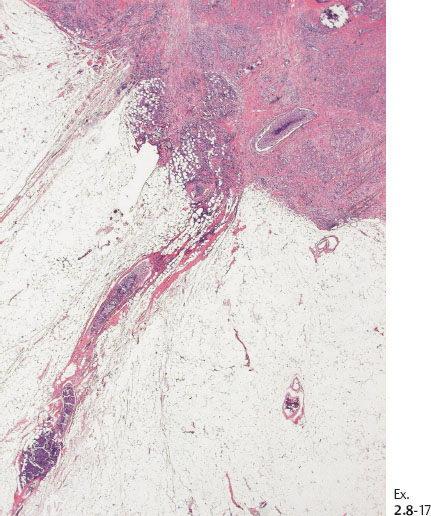
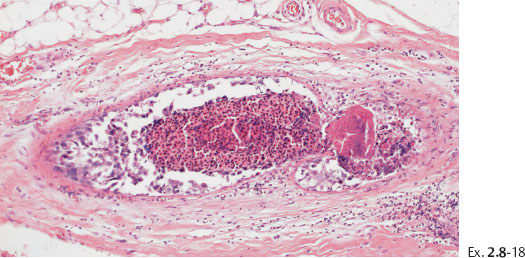
Ex. 2.8-18 Conventional histology (H&E). Higher magnification of the in situ component with calcification.
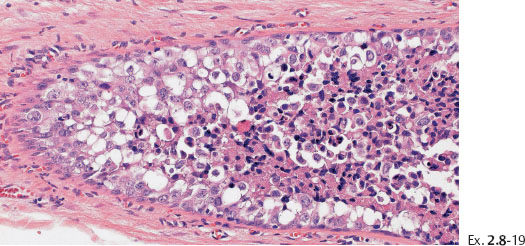
Ex. 2.8-19 Histological (H&E) detail of the in-situ carcinoma.
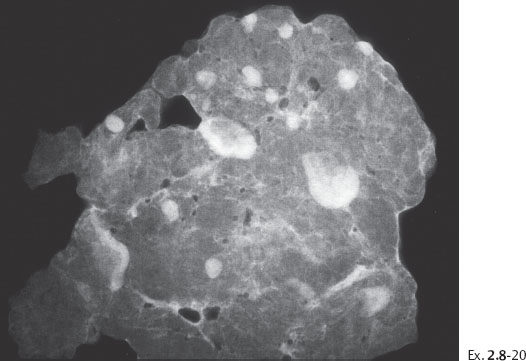
Ex. 2.8-20 Specimen radiograph of the axillary specimen containing 16 lymph nodes.
Follow-up: The patient was alive and well at the most recent examination, nine years following mastectomy.
(Case courtesy of Michael Vendrell, M.D., Saint Paul, MN, USA)
A 49-year-old asymptomatic patient had mammography and breast MRI because her sister had a breast cancer.
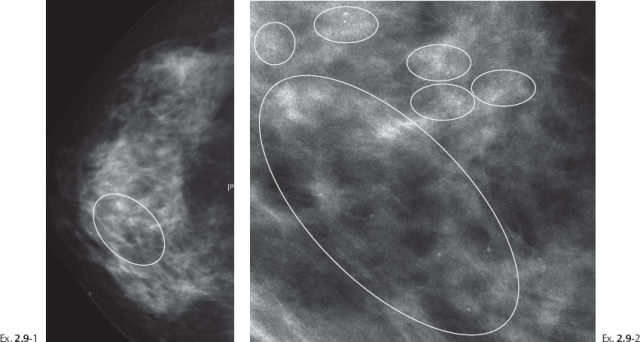
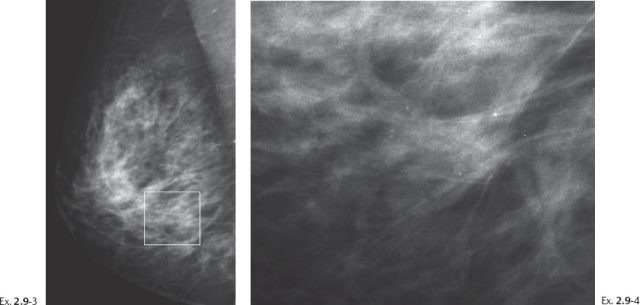
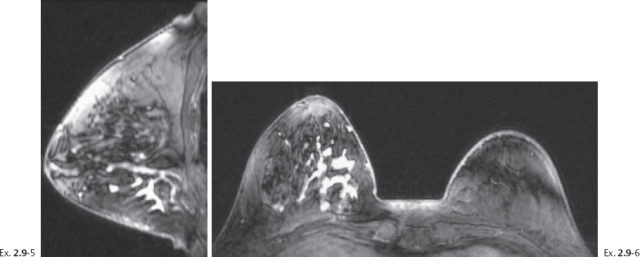
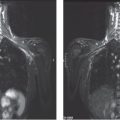
Ex. 2.9-5 & 6 Breast MRI, sagittal (5) and axial (6) planes, water excitation, post-gadolinium contrast shows irregular, ductlike enhancement in the lower inner quadrant of the right breast.
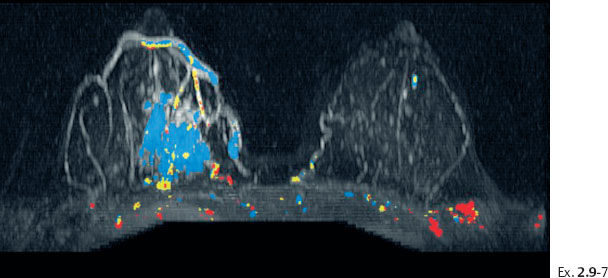
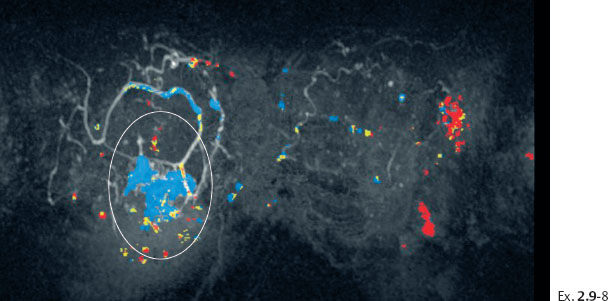
Ex. 2.9-8 Breast MRI, coronal projection, same parameters as Ex. 2.9-7. The pathological enhancement is encircled.
Histology of 14G core biopsy: Grade 3 DCIS without sizable areas of necrosis. No invasive foci were found.
Comment
These images demonstrate the ability of breast MRI to detect the disease much earlier and to a greater extent than mammography.