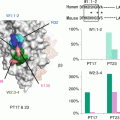
Fig. 1
Structure and binding site of romiplostim and eltrombopag. Upper panel: romiplostim is a “peptibody” and TPO receptor-binding domain consists of 14 amino acids (Ile-Glu-Gly-Pro-Thr-Leu-Arg-Gln-Trp-Leu-Ala-Ala-Arg-Ala), and eltrombopag is a nonpeptide compound. Lower panel: binding sites for TPO, romiplostim, and eltrombopag on the TPO receptor, c-Mpl. CRM cytokine receptor homology module
Romiplostim was active in mice, rats, rabbits, and monkeys and induced dose-dependent increase in platelets in all species, although nonhuman primates were less responsive compared with other tested species. The first human study was conducted in healthy volunteers based on the data obtained from rhesus monkeys. A single intravenous dose of 10 μg/kg was initially investigated in human volunteers, which was anticipated to be a no-effect dose. However, this dose was found to increase platelet count almost sixfold. This distinct effect between humans and rhesus monkeys was probably due to a much higher affinity of romiplostim for human c-Mpl than the monkey c-Mpl. A clinically effective dose that increases platelet count twofold was identified as 1 μg/kg. Intravenous (IV) administration of 1 μg/kg gave a peak of 12,900 pg/mL, while subcutaneous (SC) administration gave undetectable levels of romiplostim concentrations probably due to slow adsorption (less than 18 pg/mL). IV and SC routes produced almost identical effects in healthy volunteers and peaked at days 14–15, and in its current formulation, romiplostim is administered as SC injection every week [10, 13].
Eltrombopag (Promacta® in the United States or Revolade® in EU and Japan, SB497115; Novartis)
Eltrombopag is an orally administered, low molecular weight (546 Da), nonpeptide TPO-RA. In human studies in healthy male volunteers, single oral dose of eltrombopag did not increase platelet count. However, daily oral administration for 10 days increased platelet counts dose dependently. For subjects at the 75 mg dose, the increase in platelet count started on day 6 and peaked at days 14–16.
Eltrombopag has unique characteristics regarding in vivo activity between species. Eltrombopag showed activity in human and chimpanzee TPO receptors, but not cynomolgus monkey, mouse, or rat TPO receptors. This species difference is due mostly to a single amino acid difference in transmembrane domain of c-Mpl. Human and chimpanzee TPO receptors have a histidine at residue 499, whereas other species have a leucine 499. Exchanging of leucine 499 for histidine 499 made the cynomolgus monkey TPO receptor responsive to eltrombopag. These data suggest that the binding site of eltrombopag is not CRM1 but transmembrane domain of human TPO receptor. Thus, the binding sites of eltrombopag and rhTPO seem quite different (Fig. 1) [2].
3.2 Medical Indication and Efficacy of TPO-RAs in ITP Management
For use in adult patients with chronic ITP, both TPO-RAs have been approved in many countries. In the United States, each TPO-RA is only available for patients with “refractory” chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy and who have an increased risk of bleeding. In EU, indication of TPO-RAs is much more limited, and each drug is only available for splenectomized patients who are refractory to other treatments or may be available for nonsplenectomized patients where surgery is contraindicated [14, 15].
For children with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy, eltrombopag has been approved by the US Food and Drug Administration (FDA) in June, 2015, and by the European Commission (EC) in April, 2016 [16, 17]. Efficacy and safety for the treatment of symptomatic children ITP with romiplostim has also been demonstrated [18], and approval for child ITP is now underway.
Therapeutic goal of ITP is not to normalize the platelet count, but to elevate platelet count to a safe range (mostly above 50 × 103/μL) to minimize the risk of bleeding with minimal side effects of drugs such as corticosteroids and TPO-RAs [15]. In this context, patients with ITP were eligible if platelet counts were less than 30 × 103/μL, and they had failed at least one prior treatment in most clinical studies employing either of TPO-RA. Platelet count >50 × 103/μL by TPO-RA was considered as platelet response in most studies. These clinical studies have indicated that each drug is very effective in the treatment of chronic ITP as described below.
Romiplostim
Long-term (24 weeks) administration of romiplostim in 63 splenectomized and 62 nonsplenectomized patients with ITP was assessed in two parallel trials both of which are in a double-blind randomized controlled trial conducted in multiple centers internationally [19]. All patients were randomized 2:1 (romiplostim:placebo) to receive a starting dose of 1 μg/kg. Doses of drug were adjusted to maintain platelet count of 50–200 × 103/μL. The primary endpoints were to get a durable platelet response (platelet count ≥50 × 103/μL during 6 or more of the last 8 weeks of treatment) and treatment safety. A durable platelet response was achieved by 16 of 42 (38%) splenectomized patients with romiplostim, while that was achieved by 25 of 41 (61%) nonsplenectomized patients with romiplostim. The overall platelet response including the durable or transient platelet response was 79% (33/42) of splenectomized and 88% (36/41) of nonsplenectomized patients. In the placebo groups, 0/21 splenectomized patients and 3/21 nonsplenectomized patients responded. Mean doses of romiplostim for splenectomized patients were 4–5 μg/kg, while those for nonsplenectomized patients were approximately 3 μg/kg. Twelve of 23 (52%) splenectomized or nonsplenectomized patients with romiplostim discontinued all of their concurrent ITP drugs.
A clinical study with nonsplenectomized ITP patients suggested that as compared with standard of care, romiplostim treatment may induce greater improvement in quality of life [20].
Eltrombopag
In contrast to SC administration of romiplostim, eltrombopag is orally administrated and is absorbed with a peak concentration occurring 2–6 h after oral administration. Eltrombopag pharmacokinetics is altered when the drug is administered with polyvalent cations such as iron, calcium, magnesium, etc. Eltrombopag should be taken at least 4 h before or after any products such as antacids, dairy products or other calcium-containing food products, or mineral supplements containing polyvalent cations. In vitro studies indicate that eltrombopag is metabolized in the liver, and systemic exposure to eltrombopag is increased in patients with mild or moderate to severe hepatic impairment (41 and 80–93% increases in AUC∞, respectively). Thus, administration of eltrombopag to patients with moderate to severe hepatic impairment should be undertaken with caution and should be closely monitored [21].
Six-week administration and 6-month administration (RAISE study) of eltrombopag were assessed in phase III clinical studies in a double-blind randomized controlled trial conducted in multiple centers internationally [22, 23]. The doses for these studies were 50 mg or matching placebo once daily, and patients with platelet count <50 × 103/μL on day 22 or after may have had their dose increased to 75 mg. In addition, in RAISE dose decreases to 25 mg once daily were required for patients with platelet count >200 × 103/μL. For patients with platelet count of >400 × 103/μL, study treatment was interrupted and resumed at the next lowest dose when platelet count fell to <150 × 103/μL. The primary endpoints were the odds of achieving platelet count 50–400 × 103/μL during the 6-month treatment periods. Seventy-nine percent (106/135) of patients in the eltrombopag group responded at least once, compared with 28% (17/62) of patients in the placebo group. The odds of responding over the 6-month treatment period were greater (odds ratio = 8.2, 99% CI 3.59–18.73; p < 0.0001) for eltrombopag group. Fifty-nine percent (37/63) in the eltrombopag group reduced or discontinued baseline treatments compared with 32% (10/31) of patients in the placebo group. Thus, eltrombopag is effective for management of chronic ITP, and starting dose is 50 mg once daily for most patients and could be increased to 75 mg once daily.
Unique Pharmacokinetics of Eltrombopag in East Asian Peoples
Patient ethnicity may affect the pharmacokinetics of eltrombopag. AUC exposure to eltrombopag was approximately twofold greater among Japanese healthy volunteers than among non-Asian (predominantly Caucasian) volunteers and 87% greater among ITP patients of East Asian descent compared to non-East Asian ITP patients. From these data, it has been recommended that for patients of East Asian ancestry (such as Japanese, Korean, Chinese, Taiwanese, etc.), initiate dose of eltrombopag should be reduced to 25 mg once daily. It is noteworthy that a Japanese clinical trial evaluated the efficacy and safety of eltrombopag at a starting dose of 12.5 mg and a maximum dose of 50 mg in the treatment of Japanese patients with previously treated chronic ITP [24]. During the first 3 weeks of treatment with 12.5 mg eltrombopag, 22% (5/23) of Japanese patients responded. Since disease state is in chronic nature and 12.5 mg tablet is only available in Japan so far, it is possible that 25 mg every-other-day administration as a starting dose may be suitable for some chronic ITP patients of East Asian ancestry to prevent overshooting of platelet count.
3.3 Clinical Safety
For romiplostim and eltrombopag long-term extension studies have been being performed, respectively [25, 26]. Both TPO-RAs were generally safe and well tolerated in log-term extension studies. In romiplostim studies, headache was the most commonly reported adverse event, followed by nasopharyngitis, fatigue, contusion, upper respiratory tract infection, diarrhea, and epistaxis. In eltrombopag studies, headache was also the most common, followed by nasopharyngitis, upper respiratory tract infection, and fatigue. These common events were mostly mild. Eltrombopag may cause hepatotoxicity, and ALT, aspartate aminotransferase (AST), and bilirubin should be monitored during treatment [26].
In terms of the development of neutralizing antibodies against endogenous TPO, no patient developed such antibodies. Although two patients treated with romiplostim developed antibodies that neutralized romiplostim, but resolved after drug withdrawal, the antibodies did not cross-react with TPO or affect platelet count [13].
Since long-term treatment with TPO-RAs would be expected in chronic ITP, one should pay special attention to possible severe complications listed in Table 1.
Table 1
Possible adverse effects of TRO receptor agonists
1. | Thrombotic complications |
2. | Induction of reticulin formation in bone marrow |
3. | Increase in blast cell count |
4. | Rebound thrombocytopenia upon stopping treatment |
Thrombotic Complications
It has been suggested that ITP itself may be not only a hemorrhagic disease but also a prothrombotic disease. Population-based studies showed evidence that the risk for venous thrombosis is higher (around two time) in chronic ITP compared with controls. For arterial thrombosis there is a trend for increased risk in patients with chronic ITP, but bot statistically significant [27]. Arterial thrombotic and venous thromboembolic events have been reported for patients with both romiplostim groups and eltrombopag groups, but also in placebo groups. There is no relationship between platelet counts and thrombotic events during the treatment of TPO-RAs. Most of the patients with thromboembolic events were old age and had pre-existing risks for thrombosis (e.g., Factor V Leiden, antiphospholipid syndrome, diabetes mellitus, smoking, etc.) [25, 26]. Although it is still uncertain that TPO-RAs may apparently induce arterial thrombotic/venous thromboembolic events, special attention should be paid for patients having pre-existing risks for thrombosis when starting TPO-RAs.
Induction of Reticulin Formation in Bone Marrow
Interleukin 11, GM-CSF, and TPO agents may increase bone marrow reticulin, possibly through local release of transforming growth factor-β from the increased number of bone marrow megakaryocytes. Prolonged administration of large doses of romiplostim to mice produced bone marrow fibrosis that was reversible within 4 weeks by stopping romiplostim [28]. The presence of or increase in bone marrow reticulin was reported in some patients with long-term treatment with romiplostim, and most of them had reticulin levels of mild to moderate [29, 30]. Because of this possible adverse effect, all patients treated with TPO-RAs continue to be monitored for clinical signs of any progressive bone marrow abnormalities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree