WHO grade I
Benign meningiomas: with low risk of recurrence and/or low risk of aggressive growth
WHO grade II
Atypical meningiomas: with increased mitotic activity or three or more of the following features, increased cellularity, small cells with high nucleus-to-cytoplasm ratio, prominent nucleoli, uninterrupted patternless or sheetlike growth, and foci of spontaneous or geographic necrosis
WHO grade III
Anaplastic (malignant) meningiomas: exhibit frank histologic features of malignancy far in excess of the abnormalities present in atypical meningiomas
11.1.3 Clinical Features
Children with meningiomas often experience insidious, nonspecific symptoms and signs related to increased intracranial pressure. Therefore, they generally present with a relatively long duration of symptoms (average 6–10 months) and large tumors at diagnosis (Arivazhagan et al. 2008).
Headache (91 %) and vomiting (70 %) are the most common presenting complaints in adolescents. In infants, a tense or bulging fontanel can be seen (Amirjamshidi et al. 2000). Other patients present with distinct neurologic symptoms related to tumor location, such as motor deficits or hemiparesis (20 %) (Erdincler et al. 1998). A large number of patients develop visual deficits (51 %) or cranial neuropathies (64 %) due to the high incidence of skull base and infratentorial tumors (Arivazhagan et al. 2008). Epilepsy is relatively uncommon in the pediatric population and occurs in 20–30 % of patients (Erdincler et al. 1998; Amirjamshidi et al. 2000).
11.1.4 Diagnosis and Neuroimaging
Magnetic resonance imaging (MRI) is the mainstay of diagnosis of intracranial meningioma. Tumors show intense enhancement upon administration of gadolinium contrast agent and are frequently cystic and calcified. An enhancing dural tail can be seen (Fig. 11.1), but is less common in children than in adults. Other studies, including contrast-enhanced computed tomography (CT) scans and plain films, may serve to confirm the diagnosis. On a brain CT scan, a meningioma appears as a dural-based tumor that usually compresses, but does not invade the brain. It is usually intensely and homogenously enhancing and may be surrounded by extensive edema. On a plain radiograph, intracranial calcifications and hyperostosis may be seen.

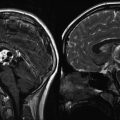
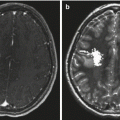
Fig. 11.1
(a) A T1-weighted MR image following contrast administration showing a dural-based convexity meningioma with an enhancing “tail.” (b) T1-weighted postcontrast MR images (axial and coronal) of a 6-year-old girl with a very unusual meningioma located in the Sylvian fissure without a defined dural attachment point
Conventional catheter angiography typically shows a delayed vascular blush and a “sunburst” pattern of feeding meningeal arteries. This modality was used in the past for diagnosis; however, it is now limited to those cases in which preoperative embolization is being considered. Catheter angiography, magnetic resonance arteriography (MRA), and magnetic resonance venography (MRV) are important tools in planning surgery by determining vascularity of the tumor, impingement on critical vascular structures, and patency of involved dural sinuses.
11.1.5 Treatment
Surgical gross total resection (GTR) is the primary treatment objective in children. In various series, GTR was accomplished in 65–86 % of cases (Erdincler et al. 1998; Tufan et al. 2005; Arivazhagan et al. 2008; Caroli et al. 2006). Specific surgical challenges in children include the proximity of the tumor to vital structures, larger tumor size at diagnosis, and lower blood volume. Multiple staged resections are sometimes necessary, particularly when significant perioperative blood loss occurs.
Preoperative embolization of meningiomas is shown to decrease tumor vascularity and reduce blood loss during surgery in adults, although there is no data specifically pertaining to children (Oka et al. 1998). Pediatric patients may have an increased risk of morbidity from this procedure due to their smaller caliber vessels.
Adjuvant chemotherapy and radiotherapy have limited roles in the treatment of pediatric meningioma, and their use varies widely between institutions. The benefits of radiation therapy must be balanced against the risks of long-term sequelae, particularly in infants and young children, whose brains are particularly vulnerable to the effects of radiation. The Children’s Cancer and Leukemia Group (CCLG) suggests consideration of radiation therapy in patients with WHO grade I and II meningiomas who experience multiple recurrences not amenable to further surgery, evidence of clinically relevant progression that threatens vital functions after subtotal resection, and in all WHO grade III tumors at diagnosis (Traunecker et al. 2008). However, a meta-analysis reported in the Lancet in 2011 showed no benefit to upfront radiation therapy, even in WHO grade III tumors, and therefore aggressive surgical management with close observation was recommended (Kotecha et al. 2011). Data on the use of stereotactic radiosurgery in children is limited, but radiosurgery has been reported as a successful treatment for recurrent tumor in a few series (Li and Zhao 2009). The use of drug therapy in the treatment of meningioma has been disappointing in adults, and therefore, chemotherapy is not routinely used in children with meningiomas.
11.1.6 Outcome
Extent of tumor resection is described as the most significant prognostic factor (Kotecha et al. 2011). Reviews of the literature show that almost all children who experienced a recurrence had a malignant histologic variant or a subtotal resection. In some series, pediatric meningiomas are reported as behaving more aggressive and carrying a worse prognosis compared with adult meningiomas (Mehta et al. 2009); however, this is controversial. In general, children with typical benign meningioma and complete removal show a good prognosis, similar to their adult counterparts.
11.2 Pediatric Pituitary Adenoma
11.2.1 Epidemiology
Pituitary adenomas account for only 1–10 % of all childhood brain tumors and between 3 % and 6 % of all surgically treated pituitary tumors (Mindermann and Wilson 1995). Variation in reported incidence is related to the lack of consensus on an age cutoff for pediatric tumors. The majority of children present with hormone-secreting tumors. Nonfunctioning adenomas are rare in children and only make up 3–6 % of tumors in this population, compared with one-third of adenomas found in adult series (Partington et al. 1994; Mindermann and Wilson 1995; Webb and Prayson 2008).
The most common functioning adenomas in children are prolactinomas (45–53 %), followed by adrenocorticotropic hormone (ACTH)-secreting adenomas or corticotroph adenomas (25–33 %), and finally growth hormone (GH)-secreting adenomas or somatotroph adenomas (8–15 %). Thyroid hormone-secreting tumors or thyrotroph adenomas are extremely rare, and only a few pediatric cases have been reported in the literature. The vast majority of cases present in adolescence; only 25 % of pediatric patients with pituitary adenomas are under the age of 12 (Partington et al. 1994; Mindermann and Wilson 1995). When stratified into prepubescent (age 0–11), pubescent (age 12–17), and postpubescent (age 18–19) groups, there is a characteristic distribution by tumor type (Table 11.2). Corticotroph adenomas are most commonly seen in the youngest age group, found in over 50 % of prepubescent children diagnosed with pituitary adenomas. Somatotroph adenomas are equally distributed among the three age groups. The distribution of the various adenomas in the postpubescent group mimics that in adults.
Adenoma subtype | No. of patients (%) | |||
|---|---|---|---|---|
Age 0–11 | Age 12–17 | Age 18–19 | Total | |
Prolactinoma | 5 (16.1) | 61 (59.8) | 12 (70.6) | 78 (52.0) |
Corticotroph adenoma | 22 (71.0) | 31 (30.4) | 3 (17.6) | 56 (37.3) |
Somatotroph adenoma | 2 (6.4) | 8 (7.8) | 2 (11.8) | 12 (8.0) |
Endocrine inactive | 2 (6.4) | 2 (2.0) | 0 | 4 (2.7) |
Totala | 31 (20.7) | 102 (68.0) | 17 (11.3) | 150 (100) |
Multiple large series have shown a female preponderance in pediatric pituitary adenomas (Maira and Anile 1990; Kane et al. 1994; Mindermann and Wilson 1995; Webb and Prayson 2008). The vast majority of prolactinomas occur in females (80 % females vs. 20 % males), and slightly more corticotroph adenomas occur in females than in males. On the other hand, pure somatotroph adenomas are more common in males.
11.2.2 Histopathology
Most pituitary adenomas are benign tumors arising from epithelial cells of the adenohypophysis. Tumor development occurs as a monoclonal process influenced by a multitude of factors, including hormones, gene mutations, or heredity. The exact pathophysiologic mechanism, however, is unknown. Pituitary adenomas can occur sporadically or as components of hereditary syndromes. Prolactinomas, corticotroph adenomas, and somatotroph adenomas are common in patients with multiple endocrine neoplasia syndrome type 1 (MEN-1). Somatotroph adenomas are also associated with several other hereditary conditions, including McCune Albright syndrome or the Carney complex (Lafferty and Chrousos 1999).
There is some discrepancy among studies regarding the ratio of macroadenomas (>1 cm) to microadenomas. Most studies document either equal distribution between the two types or a slightly higher incidence of macroadenomas in the pediatric population (range 36–78 %). The size of the tumor appears to be related to secretory function, with the vast majority of somatotroph and thyrotroph adenomas presenting as macroadenomas and corticotroph adenomas presenting as microadenomas. Hormonally inactive adenomas often present as macroadenomas.
11.2.3 Clinical Features
Presenting symptoms in patients with pituitary adenomas depend both on tumor size and on secretory capability. Headaches and visual deficits are common in children with macroadenomas. Because pediatric patients have a preponderance for functioning adenomas, the majority of children also present with endocrine dysfunction (75 %) (Pandey et al. 2005). This dysfunction manifests as specific clinical signs related to hormone hypersecretion from the tumor, as well as disruptions in growth or sexual maturation related to compression of normal pituitary tissue. The pituitary gland shows a predictable sequence of secretory failure (Kunwar and Wilson 1999). GH-releasing cells are extremely vulnerable to compression and are almost always the first cells to exhibit hormone hyposecretion. Therefore, children with adenomas other than somatotroph adenomas usually present with short stature or growth retardation. Gonadotropin-releasing cells are also susceptible to compression, although to a lesser degree than GH-secreting cells, and menstrual irregularity is a common complaint in adolescent girls with pituitary adenomas. Thyroid-stimulating hormone (TSH) is affected late and only with relatively large tumors.
Prolactinomas can present with different signs and symptoms depending on the age and sex of the child. Prepubertal children present with nonspecific symptoms of headache, visual disturbances, and growth failure, mentioned earlier. Common complaints by postpubertal females are amenorrhea and galactorrhea. These tumors are rare in males, but may present with pubertal arrest, hypogonadism, or gynecomastia. Because prolactinomas arise from cells in the same lineage as somatotrophs and thyrotrophs, these tumors may also stain for and secrete GH and TSH.
Cushing’s syndrome is the most common presentation of corticotroph adenomas. Approximately 90 % of children over the age of 5 years diagnosed with Cushing’s syndrome have ACTH-secreting adenomas (Lafferty and Chrousos 1999). These patients experience weight gain (50 %) with purplish striae, easy bruising, moon facies, growth retardation, and sometimes hypertension and insulin resistance. Prepubertal children may present with hirsutism and premature pubarche, while postpubertal children often have pubertal arrest. Unlike adults, proximal myopathy is uncommon in children, and weight gain tends to be generalized rather than centripetal (Lafferty and Chrousos 1999). Neuropsychiatric problems such as compulsive behavior and overachievement in school tend to dominate in the pediatric population, as opposed to depression and memory loss which are common in adults.
Somatotroph adenomas generally cause rapid growth and gigantism in children with open epiphyseal plates. Occasionally, these tumors also cause weight gain, premature puberty, or menstrual irregularities. In older children, who have undergone epiphyseal fusion, symptoms tend to mimic those in adults. These patients may present with glucose intolerance, acromegalic features such as coarse facies and enlarged hands and feet, nausea, and respiratory difficulty.
Nonfunctioning adenomas, which are uncommon in children, present with generalized symptoms of headache, visual disturbance, growth retardation, and pubertal arrest. Thyrotroph adenomas, which are exceedingly rare, may present with hyperthyroidism or nonspecific findings similar to those patients with nonfunctioning adenomas.
11.2.4 Diagnosis and Neuroimaging
11.2.4.1 Imaging
Pituitary adenomas are diagnosed by both imaging and biochemical studies. MRI with gadolinium is the imaging modality of choice and has 72 % sensitivity in the diagnosis of adenomas (Devoe et al. 1997). On T1-weighted images, pituitary adenomas are hypoenhancing, compared with normal anterior pituitary tissue that appears slightly hyperintense relative to the rest of the brain (Fig. 11.2). Nonspecific signs may include a deviation of the pituitary stalk or an increase in the vertical height of the gland, although a normal pituitary gland in adolescent girls may appear enlarged and convex superiorly. Physiologic enlargement of the pituitary gland during puberty is frequently mistaken for a macroadenoma. Although MRI provides superior soft tissue contrast and resolution of the sella compared to CT, MRI is still only able to detect less than one-half of microadenomas.
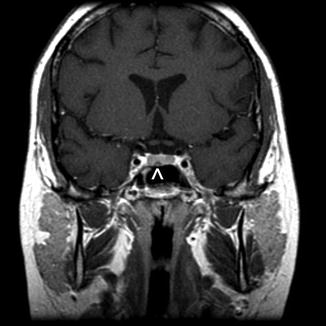

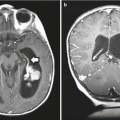
Fig. 11.2
A teenage girl presenting with Cushing’s disease secondary to a hormone-secreting corticotroph microadenoma. A T1-weighted coronal MR image shows a small lesion (arrowhead) that is hypointense relative to the pituitary gland. The normal pituitary gland is usually brighter than a small pituitary adenoma following contrast
Positron emission tomography (PET) scans and ultrasound may be useful in some cases, although these modalities have not been used yet in primary diagnosis. Ultrasound has shown promise during transsphenoidal surgery, particularly in patients with corticotroph adenomas (Ram et al. 1999). This modality provides good contrast between tumor and normal pituitary gland and has the potential to identify sellar or suprasellar structures with invasion or damage. In postoperative patients, PET can help differentiate recurrence from scarring.
11.2.4.2 Biochemical Tests
Hematologic tests depend on clinical presentation. Prolactin levels are checked in any patient with a suspected prolactinoma. A single elevated level of greater than 200 in a patient with a 1 cm pituitary mass on imaging is adequate for a diagnosis of prolactinoma. Because moderately elevated levels can signify either a microadenoma or secondary hyperprolactinemia due to other causes such as functional pituitary stalk disconnection, a second, fasting level may be checked for confirmation and is usually drawn an hour after placing an indwelling cannula.
Suspected corticotroph adenomas warrant a workup that confirms the presence of Cushing’s disease. Multiple 24 h urine free cortisol (UFC) levels are checked and adjusted for the child’s body surface area. In addition, a dexamethasone suppression test can be administered and is positive for Cushing’s disease if the patient’s serum cortisol fails to drop the morning after receiving a dose of dexamethasone. Once Cushing’s disease is diagnosed, further tests are ordered to confirm that the etiology is a corticotroph adenoma. A high-dose dexamethasone suppression test is positive if a patient’s serum cortisol drops by 50 % the morning after a high dose of dexamethasone is administered and has 85 % sensitivity for diagnosis of corticotroph adenoma (Lafferty and Chrousos 1999). Additional tests involve injecting ovine corticotropin-releasing hormone and measuring ACTH simultaneously from central and peripheral sites. If ACTH is stimulated, the test has 97 % sensitivity for corticotroph adenoma. Alternatively, bilateral inferior petrosal sinuses can be sampled, and detection of ACTH both confirms the diagnosis and lateralizes the tumor with 75 % accuracy.
Biochemical tests for somatotroph adenomas include random measurements of GH and insulin-like growth factor (IGF-1). Elevations in both markers suggest a diagnosis of somatotroph adenoma, although IGF-1 can sometimes be elevated during normal puberty. An oral glucose tolerance test, during which GH shows failed suppression or a paradoxical rise, is also positive for a somatotroph adenoma. This test, however, has a high false-positive rate (Holl et al. 1999) and should be used in conjunction with other laboratory testing. Somatotroph adenomas also frequently express thyrotropin-releasing hormone (TRH) receptors, and therefore, the diagnosis can be confirmed by measuring GH stimulation after TRH administration.
Several tests may be used to help confirm the presence of a thyrotroph adenoma. Elevated free T4 and T3 without suppression of TSH support the diagnosis. However, additional tests differentiate an adenoma from central T4 resistance. Thyrotroph adenomas do not respond to TRH stimulation, and this test has a sensitivity of 71 % and specificity of 96 %. In addition, elevated alpha-glycoprotein subunit is a positive test and has 75 % sensitivity and 90 % specificity.
11.2.5 Treatment
11.2.5.1 Surgery
Surgical treatment for pituitary adenomas has been widely used during the past 50 years. Surgery has the advantage of rapidly lowering hormone levels in functioning adenomas and improving visual symptoms in patients with optic chiasm compression. Two general surgical approaches are used: transcranial/subfrontal or transsphenoidal. In some situations with very large tumors, a combination of approaches is needed. At present, the preferred technique is the transsphenoidal route in both adults and children. In several series, transsphenoidal surgery was used in children as the initial approach. This technique yielded positive results, and very few patients suffered postoperative complications or required a second intracranial procedure (Maira and Anile 1990; Partington et al. 1994). The transsphenoidal route has been used in children as young as 4 years old without difficulty (Haddad et al. 1991; Ludecke and Abe 2006). In patients with larger tumors, the transcranial route is often used. Specific indications include dumbbell-shaped tumors with extensive suprasellar component, multi-compartmental tumors, and unclear diagnoses (Pandey et al. 2005). However, morbidity is often higher in patients operated on via this route, and postoperative visual deterioration is more common.
Surgery as primary therapy is recommended for all pituitary adenomas except prolactinomas. Corticotroph adenomas usually present as microadenomas, and therefore, hemihypophysectomy is often curative. However, this approach entails confirmation of the correct half of the anterior pituitary gland containing the microadenoma. In patients with functional corticotroph or somatotroph adenomas, 80 % will be cured following surgery alone (Mindermann and Wilson 1995). Although surgery is the preferred treatment for thyrotroph adenomas, these tumors are often large and invasive and therefore require adjuvant radiation therapy (Kunwar and Wilson 1999). While adult patients with asymptomatic, nonfunctioning adenomas are usually observed, pediatric patients are almost always treated surgically. This approach is primarily due to the fact that nonfunctioning adenomas are extremely rare in children and difficult to discern from craniopharyngiomas.
In children with prolactinomas, medical rather than surgical therapy is recommended as the initial treatment (CasaNueva et al. 2006). Surgery is reserved for individuals who have rapid visual decline (Fig. 11.3), are intolerant of the side effects of medication, or are unwilling to comply with lifelong pharmacologic therapy. Some studies do report good results in pediatric patients who undergo surgery for prolactin-secreting tumors; one large series reported an 82 % cure rate (Mindermann and Wilson 1995).
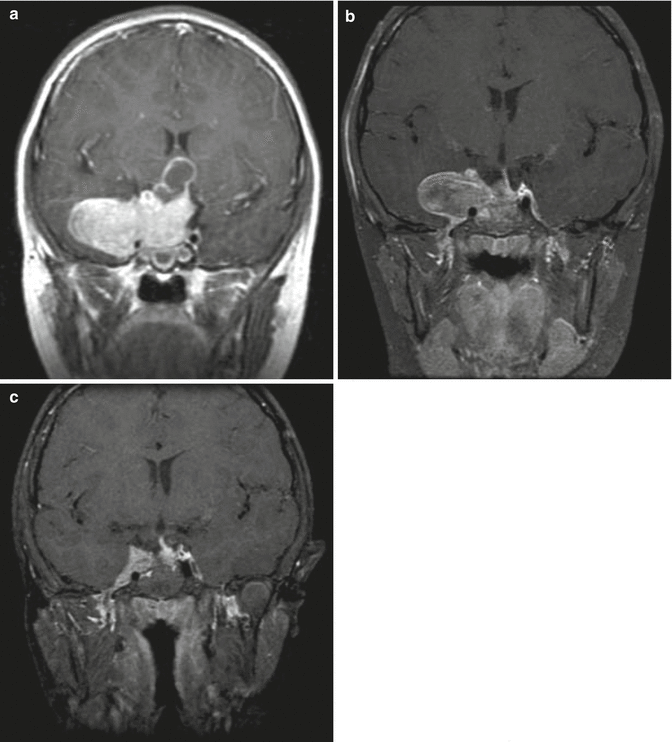

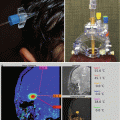
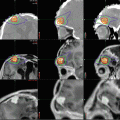
Fig. 11.3




A 5-year-old boy who presented with visual loss from a very large prolactinoma. The tumor was treated with subtotal surgical resection to decompress the optic chiasm and preserve visual function. The preoperative T1-weighted coronal MR image (a) shows a large multilobulated mass with poor visualization of the chiasm. The immediate postoperative scan (b) shows excellent decompression of the suprasellar tumor although the lateral portion remains. Two years later (c), after taking oral cabergoline, a much smaller residual tumor remains adjacent to the cavernous sinus. The patient has a partial GH deficiency, but otherwise has normal visual and endocrine function
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree