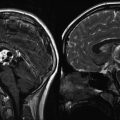
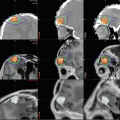
Fig. 9.1
A large choroid plexus papilloma. (a) A sagittal precontrast T1-weighted MRI demonstrates a large lobulated mass within the lateral ventricle with associated enlargement of the ventricle. (b, c) Following contrast, the mass enhances brightly. Note that the papilloma is well demarcated from the ventricular wall

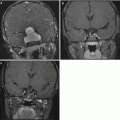
Fig. 9.2
(a) An axial postcontrast image shows a large choroid plexus papilloma within the temporal horn of the lateral ventricle. (b) The perfusion sequence results in a “negative” image with increased vascularity depicted as a dark area. The mass is considerably darker than the adjacent brain tissue

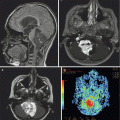
Fig. 9.3
(a) A coronal postcontrast MR image showing a large parietal choroid plexus carcinoma. The vascular pedicle can be seen at its most inferior portion adjacent to the atrium of the lateral ventricle. (b) An axial T2-weighted image showing the extent of the mass effect and peritumoral edema
9.6 Treatment
9.6.1 Preoperative Planning and Treatment
Since most patients present with symptoms of intracranial hypertension, treatment of hydrocephalus goes along with planning for the actual tumor resection. Unless the patient is rapidly deteriorating, urgent CSF drainage is not necessary. At the time of surgery, a ventricular drain is usually placed in order to reduce brain tension and allow sufficient retraction. An external ventricular drain may be left in place after surgery in order to monitor ICP and to determine if shunting is required in the early postoperative period. Matson and others have reported that the successful removal of a tumor obviates the need for shunting. However, it is likely that other factors such as ventricular bleeding, postoperative changes, or meningitis can also render the patient shunt dependent. Ellenbogen’s series noted that 37 % of surviving patients required shunting (Ellenbogen et al. 1989). Two other series reported much higher rates of shunt dependency, ranging from 57 % to 78 % of cases reported (Humphreys et al. 1987; Lena et al. 1990). There is some evidence that patients with larger tumors, or hydrocephalus present prior to surgery, are at higher risk for requiring permanent CSF diversion (Safaee et al. 2013 #154).
Conventional catheter angiography is not required for diagnosis. Rather, its primary role is as a preoperative adjunct to define the blood supply and can be combined with embolization to reduce tumor vascularity. Angiography clearly indicates that the vascular supply of papillomas is from normal choroidal vessels, which often enlarge as the tumor grows. Tumors of the lateral ventricle or third ventricle are generally supplied by branches of the anterior or posterior choroidal arteries. Mass effect tends to displace the internal occipital artery and the basal vein of Rosenthal in an inferior direction. A fourth ventricle tumor receives its blood supply from medullary or vermian branches of the posteroinferior cerebellar artery.
9.6.2 Operative Treatment
The goal of surgery is gross total resection (GTR), as measured by postoperative MR imaging. As with most intracranial tumors, the exact approach is determined by avoiding eloquent tissue (primary motor or sensory cortex, speech centers, and visual cortex). The two features of choroid plexus tumors that can make resection exceedingly difficult are (1) profuse vascularity and (2) large size. The tumor’s arterial vessels arborize rapidly, and so control of hemorrhage within the tumor requires slow and tedious dissection. The most effective strategy focuses on initial exposure of the feeding artery and its ligation (Fig. 9.4). In general, en bloc excision is recommended (Raimondi and Gutierrez 1975). For lateral ventricle papillomas, a cerebral incision posterior to the angular gyrus allows access to the entire trigone and permits the pedicle of the tumor to be identified and coagulated. For more anteriorly located tumors, an incision can be made in the frontal convolutions and the lateral ventricle approached from an anterolateral direction. Lateral ventricle tumors can also be approached through a cerebrotomy through the superior or middle temporal gyrus.

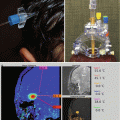
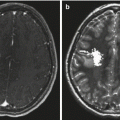
Fig. 9.4
The same case as shown in Fig. 9.1. (a) The axial T1-weighted image clearly shows an enlarged choroidal artery leading into the tumor. (b) The postoperative coronal MRI image shows the route through the temporal lobe used to access initially the feeding artery and then the tumor itself. Once the blood supply was interrupted, the tumor removal proceeded uneventfully
Third ventricle tumors are rare and are approached usually through a midline transcallosal route. The anterior aspect of the ventricle is entered through a generous opening in the corpus callosum extending from the rostrum to the supraoptic recess. In this way, the tumor can be separated from the choroid of the tela choroidea where it is usually attached and the accompanying bridging vessels can be identified and divided.
Fourth ventricle tumors almost always produce triventricular obstructive hydrocephalus and may require preoperative shunting and stabilization as noted earlier. Tumors in this location arise from the caudal part of the roof of the fourth ventricle and may extend into the lateral recesses, or through the foramen of Magendie. The approach is through a standard midline posterior fossa craniotomy exposing the vermis and tonsils. The blood supply from branches of the PICA is visualized from a medial vantage.
9.6.3 Choroid Plexus Carcinomas
In a recent systematic review, GTR clearly had a favorable impact upon survival for CPCs (Sun et al 2014). Nevertheless, GTR with carcinoma is achieved in less than 50 % of cases. Combined with adjunctive therapy, either radiation or chemotherapy, survival following GTR ranges from 67 % to 91 % (Fitzpatrick et al. 2002). Technical considerations with CPC include the expected increased tumor vascularity as well as additional difficulties relating to the lack of a well-developed plane between the brain and tumor and excessive friability of the tumor tissue. The rate of recurrence associated with GTR alone suggests that adjunctive therapy is useful, although definitive guidelines are not available (Fitzpatrick et al. 2002; Sun et al. 2014).
Most chemotherapy regimens rely upon cyclophosphamide, etoposide, vincristine, and a platinum agent (St Clair et al. 1991; Packer et al. 1992; Berger et al. 1998). Wolff et al. noted that only 8 of 22 carcinomas responded to chemotherapy, a disappointing observation (Wolff et al. 2002). Use of combination chemotherapy (ifosfamide, carboplatin, and etoposide) after an initial surgical procedure was found to reduce tumor volume and allow a more complete resection during a second-stage operation (St Clair et al. 1991; Razzaq and Cohen 1997; Lafay-Cousin et al. 2011). Importantly, the vascularity of the tumor appeared to be greatly reduced, as measured blood loss during the second procedure was on an average 15 % of blood volume compared to an average of 64 % of blood volume during the first procedure. Recent meta-analyses have noted that administration of chemotherapy resulted in a survival advantage for patients with completely or incompletely resected carcinomas and that second-look surgery is of benefit for those patients with incompletely resected CPCs (Wrede et al. 2005, 2007). Chemotherapy was also beneficial in the subgroup of patients who did not receive radiation. These observations, although retrospective in nature, suggest that aggressive therapy including chemotherapy and further attempts to remove any remaining tumor should be pursued when possible.
Postoperative radiation is usually recommended if the child is over 3 years of age, although this therapy has not been subjected to a clinical trial. Radiation is also used in the presence of leptomeningeal dissemination, subtotal resection (STR), and drop metastases. In one series, ten patients with CPC were treated with either chemotherapy or craniospinal radiation (Chow et al. 1999). Some of these patients demonstrated no evidence of disease following chemotherapy alone, but others required radiation to achieve disease control. The authors do suggest that radiation can be used as salvage therapy, but whether radiation for all patients with carcinoma would reduce the relapse rate remains unclear. Certainly, this should be judiciously used in children under 3 years of age. Fitzpatrick et al. noted that following STR, radiation therapy, either alone or in combination with chemotherapy, offered a survival advantage (Fitzpatrick et al. 2002). The question of which adjunctive therapy to use following GTR remains unclear, although the presence of relapse despite chemotherapy and radiation suggests that surgery alone is not sufficient for CPC. Wolff et al. support this view and state that GTR alone is insufficient for carcinoma and should be supplemented with radiation (Wolff et al. 1999). The role of conformal radiation and radiosurgery is unknown, nor is the role of intrathecal chemotherapy. The experience reported by Packer suggests that disease relapse confers a poor prognosis (Packer et al. 1992).
9.7 Outcome
Almost all patients with CPP can expect an excellent long-term survival. The survival for CPC, however, is much worse. In one meta-analysis, the 1-, 5-, and 10-year survival for CPP was 90, 81, and 77 %, compared to only 71, 41, and 35 % for CPC (Wolff et al. 2002). A prospective international study reported 5-year overall survival of 100 %, 89 %, and 36 % for CPPs, atypical CPPs, and CPCs, respectively. In another large series of grade I CPPs, only 12 of 124 patients recurred during a mean follow-up period of 59 months (Jeibmann et al. 2007). Of the 124 papillomas, 21 were described as having atypical histology. Six of these 21 tumors recurred, compared to 6 of the 103 tumors with normal histology. In the same series, 2 of the 103 papillomas progressed to carcinomas, which is a rare event. The extent of surgery is the most important treatment variable impacting long-term survival for both papilloma and carcinoma patients (Ellenbogen et al. 1989; Packer et al. 1992; Wolff et al. 2002). The overall survival rate in Ellenbogen’s series was 88 % for patients with CPP and 50 % for those with carcinomas (Ellenbogen et al. 1989).
Packer et al. reported that GTR for carcinoma without adjuvant therapy offers the highest likelihood of success (Packer et al. 1992). Four of five patients who underwent GTR remained disease-free at a median of 45 months after diagnosis. Five of six patients who had an STR suffered a relapse. Two other reports, however, noted that 5-year survival following GTR of carcinomas ranged from 26 % to 40 % (Berger et al. 1998; Pencalet et al. 1998). Berger et al. also noted that surgery was the most important prognostic factor for CPC. The meta-analysis by Wrede et al. confirmed the utility of chemotherapy and/or radiation for CPC (Wrede et al. 2007). A brief report noted that the 5-year survival for patients with carcinoma who were treated with GTR followed by radiation was 68 %, compared to 16 % for those not irradiated (Wolff et al. 1999). The two groups were not exactly comparable, but the clear suggestion is that surgery alone is insufficient to prevent recurrence of carcinomas.
Although papillomas are histologically benign and potentially curable, morbidity and mortality are significant concerns. With respect to operative mortality, modern series provide figures of 8–9.5 % (Humphreys et al. 1987; Lena et al. 1990). In the series from the Hospital for Sick Children, the cumulative mortality was 36 %, the majority of which (six of eight) occurred in patients below 12 months of age. Morbidity remains an important problem. In one series 33 % of patients with papillomas had persisting motor sequelae and psychomotor retardation (Lena et al. 1990). In another series, 26 % of patients were classified as having a fair or poor recovery (Ellenbogen et al. 1989).
Tabori’s analysis of CPC outcome as a function of TP53 status was striking (Tabori et al. 2010). Of the 26 patients with CPC, 38 % had immunopositivity for TP53 which correlated strongly with TP53 mutations. The overall 5-year survival for patients with TP53 immunonegativity was 82 % as compared to 0 % for patients with TP53 immunopositivity. A more detailed analysis of a 100 patients revealed subgroups within CPC, although patients with more than two copies of mutant TP53 had worse overall survival (Merino et al. 2015). These data appear to support a strong genetic determinant of tumor phenotype. These data suggest that patients in higher risk groups should be treated more aggressively.
As noted earlier, the treatment of hydrocephalus goes hand-in-hand with the treatment of choroid neoplasms, and associated complications can occur. One significant complication is the presence of large subdural collections that may develop following tumor resection, caused by a persistent venticulosubdural fistula. Boyd and Steinbok appear to have dealt with this problem by applying pial sutures at the conclusion of the procedure (Boyd and Steinbok 1987). The role of preoperative shunting in the causation of this entity is unclear.
Conclusions
Choroid plexus tumors represent a rare but well-defined subset of brain tumors that occur mainly in young children. Surgical resection for papilloma is usually curative, while adjuvant therapy for carcinoma should include chemotherapy and/or radiation. The long-term survival for carcinoma remains poor, particularly for those tumors with TP53 mutations. The overall functional outcome can be excellent, but the potential for neurologic morbidity should be recognized early even for benign tumors.
References
Arbeit JM, Munger K, Howley PM, Hanahan D (1993) Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6/E7 ORFs. Am J Pathol 142:1187–1197PubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree