Variable
Typical carcinoid
Atypical carcinoid
Large cell NEC
Small cell NEC
Mitoses per 2 mm2 (10HPF)
<2
2–10
≥10
≥10
Necrosis
Absent
Often punctate
Often, large zones
Frequent, large zones
Histologic grade
Low
Intermediate
High
High
Nuclear-cytoplasmic ratio
Moderate
Moderate
Low
High
Nucleoli
Occasional
Common
Very common
Absent/inconspicuous
Nuclear Chromatin
Finely granular
Finely granular
Usually vesicular, may be finely granular
Finely granular
Conversely, thymic NETs are considered to occur as a result of neuroendocrine differentiation of a preexistent epithelial tumor rather than as a de novo process. Also, these tumors bear a significant risk for recurrence, metastasis, and tumor-associated deaths despite presence of benign-looking features. Hence it has been suggested that these tumors be referred to as well-differentiated or poorly differentiated neuroendocrine carcinomas which are further subclassified based on specific histologic features [24] (Table 17.2).
Table 17.2
Histologic classification of thymic NETs
Neuroendocrine carcinoma | ||||
|---|---|---|---|---|
Well differentiated | Poorly differentiated | |||
Typical carcinoid | Atypical carcinoid | Large cell | Small cell | |
Mitotic index (10HPF) | <2 | 2–10 | >10 | |
Necrosis | Absent | Present | – | – |
Cytomorphology | Non-small cell | Small cell | ||
17.5 Clinical Presentation
Bronchopulmonary carcinoids can present both as intrabronchial masses and intraparenchymal pulmonary nodules. Owing to their high vascularity and tendency toward causing bronchial obstruction, the affected patients commonly present with cough, hemoptysis, and obstructive pneumonia [15]. However, tumors that are more peripherally situated are diagnosed as incidental radiologic findings. Nearly half of all the bronchopulmonary NETs have been diagnosed incidentally in asymptomatic conditions [2]. On the other hand, thymic NETs, typically the poorly differentiated NETs and about half of the well-differentiated NETs, present with local symptoms such as chest pain, cough, dyspnea, and superior vena cava syndrome [4, 5, 25].
The features of carcinoid syndrome appear quite rare in both bronchopulmonary [15] and thymic NETs [25] as many of these tumors do not secrete vasoactive substances. However, thymic NETs not uncommonly can present with an ectopic Cushing’s syndrome owing to ACTH secretion [26, 27]. Rare reports of ectopic Cushing’s syndrome have also been noted with atypical pulmonary carcinoids [28].
Thymic NETs are also known to be associated with MEN-1 (multiple endocrine neoplasia, type 1) syndrome [8, 29], in which case, the tumors are insidious and can manifest by local symptoms, metastases, disturbances of calcium/phosphate metabolism [10], or, rarely, with acromegaly [30]; however, manifestation in the form of an ectopic Cushing’s syndrome is yet to be reported in such cases.
17.6 Conventional Diagnostic Approaches
Histopathologic examination is the gold standard for the diagnosis of NETs. Invasive diagnostic modalities such as flexible bronchoscopy and thoracotomy not only help in characterizing the tumor but are also routinely used for extracting tissue samples for diagnosis by means of washings, needle aspiration, and biopsy. However, the high vascularity of these tumors comes with a caveat that these procedures can sometimes result in severe or even life-threatening hemorrhage.
Contrast-enhanced CT is the conventional imaging modality routinely used for the diagnosis of bronchopulmonary and thymic NETs. Owing to their highly vascular nature, these tumors enhance exquisitely on administration of intravenous CT contrast. However, literature suggests that thymic NETs associated with ectopic Cushing’s syndrome are comparatively small in size and may be easily missed on contrast-enhanced CT studies. Other structural imaging investigations such as chest radiography and MRI can also be used. For example, MR imaging may be used in suspect peripheral pulmonary nodules to differentiate between small carcinoids and adjacent vascular structures [31]. However, each of these modalities suffers from its own drawbacks. Also, the findings are rather nonspecific enough in establishing a diagnosis [31]. More importantly, they cannot assess the functional status of the tumor and cannot differentiate between typical and atypical carcinoids [32].
17.7 Treatment
Surgical resection is the treatment of choice for bronchopulmonary carcinoids, with lobectomy being the surgical procedure of choice for most tumors; however, limited resection maybe attempted in more peripherally situated lesion [33]. Also, as most of the poorly differentiated NETs present with locally advanced disease and tend to metastasize early, chemotherapy and radiotherapy in an adjuvant or neo-adjuvant setting are rendered necessary in these cases. However, no robust evidence is available that definitively demonstrate a benefit in the outcome [34, 35]. The prospect of theranosis has evolved into an attractive treatment option in advanced unresectable and metastatic tumors, specifically the more differentiated varieties that express somatostatin receptors [discussed later].
Thymic NETs are often aggressive in their clinical behavior. Hence, a complete resection often accompanied by mediastinal lymphadenectomy by median sternotomy approach is considered to provide the best opportunity of a curative disease removal and longer disease-free survival. Currently there are no data to suggest that an adjuvant therapy (radiation, chemotherapy, or chemoradiation) will prolong the disease-free interval or median survival [36–38].
17.8 Radionuclide Imaging of NETs
Wide arrays of SPECT and PET radiopharmaceuticals have been used in the evaluation and management of neuroendocrine tumors. In general, functional imaging with PET radiopharmaceuticals carries a higher detection rates for lesions in these patients owing to inherently better spatial resolution and a broader spectrum of radiopharmaceuticals available for targeting the various molecular mechanisms for identifying these lesions.
17.8.1 Radionuclide Imaging in Bronchopulmonary NETs
Bronchopulmonary NETs, as is the case with carcinoid tumors elsewhere in the body, express somatostatin receptors which can be used to functionally assess the various aspects in the management of these tumors. Functional imaging techniques such as somatostatin receptor and dopamine receptor imaging offer a higher sensitivity in disease detection in comparison with anatomical imaging techniques such as CT and MRI which offer sensitivities of only about 50–80 % [39].
Somatostatin receptor is an important cellular marker available in characterizing cells of neuroendocrine origin. This group of receptor consists of 7-transmembrane G-protein-coupled receptors; although five subtypes have been identified, neuroendocrine tumors are known to express predominantly the receptor subtypes 2 and 5 [40].
17.8.1.1 Somatostatin Receptor Scintigraphy
Neuroendocrine tumors are known to express somatostatin receptors (SSRs) on their cell surface. Somatostatin (SS) by itself has a very short half-life of 1–2 min and hence has no clinical application. Octreotide analogues have a high affinity for the SSR-2 and SSR-5 subtypes and a much lower binding to the SSR-1, SSR-3, and SSR-4 subtypes [41].
111In-Pentetreotide Scintigraphy
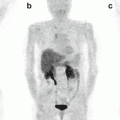
The cyclic octapeptide octreotide was the first SS analogue to be used in clinical practice. This compound was initially conjugated with diethylene-triamine-pentaacetic acid (DTPA) and then coupled with various radioisotopes especially 111In. The sensitivity of this scintigraphy technique has been reported to be about 80–90 %. Positivity on 111In-DTPA-octreotide scintigraphy (octreoscan) not only helps to localize the tumor but also helps to predict the response to octreotide therapy. Octreotide can also be conjugated with the macrocyclic chelator DOTA (1,4,7,10-tetraazacyclododecane-N,N=,N,N-tetraacetic acid), resulting in 111In 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-lanreotide (111In-DOTA-lanreotide) and 111In-DOTA-Tyr3-octreotide and enabling its use in the diagnosis, staging, and follow-up of patients with NETs [41]. This technique provides whole-body screening and is associated with radiation exposure comparable to that of other imaging modalities.
Sensitivity of SRS in different tumor types is related to various factors such as type and density of SS receptors expressed by the tumor, target-to-background ratio, and tumor site and the histology. In clinical practice, SRS is mainly used for localizing the primary lesion and for evaluating disease extension, monitoring the effects of treatment, as well as a prognostic parameter in predicting the response to therapy.
In a retrospective analysis of pulmonary carcinoids with 111In-octreotide scintigraphy, Yellin et al. [42] reported sensitivity of 90 % for SRS. The specificity was 83 % and the positive and negative predictive values were 83 % and 91 %, respectively. Hervás Benito et al. [43] evaluated the role of octreoscan in three cases of pediatric bronchial carcinoids (one preoperative, two postoperative with residual tumor); uptake was noted in all the three cases, which were later confirmed histologically. In one case the scan was able to differentiate between the tumor and associated atelectasis.
Irrespective of the promising results provided above, octreoscan carries a few but troublesome limitations such as limited spatial resolution, low tumor-to-background ratio which might hamper visualization of smaller lesions, and a high false-positive rates due to uptake in many other tumors, granulomas, and some autoimmune disease, thus reducing its specificity.
123I/131I-MIBG (MIBG Scan)
MIBG (meta-iodobenzylguanidine) is an analogue of biogenic amine precursor resembling adrenergic neurotransmitter norepinephrine. It is taken up by active sodium and energy-dependent uptake mechanism in the cell membrane of sympathomedullary tissue and is stored in the intracellular granules [44]. This molecule can be labelled with either 123I or 131I, with 123I-labelled molecule having better characteristics such as superior spatial resolution and absence of high-energy beta emissions that are emitted from 131I. On the other hand, 131I-MIBG can be a useful cost-effective option as it is much less expensive compared to 123I-MIBG.
The use of 131I-MIBG for the detection and imaging of neuroendocrine tumors was reported as early as the 1980s, followed by papers about its therapeutic applications. The sensitivity of the MIBG scan for carcinoid tumors is reported to be a little lower than that of the 111In-pentetreotide scintigraphy scan; however, a combination of these scans increases the sensitivity to 95 % [45]. MIBG scintigraphy may provide advantages over octreoscan in preoperative localization of tumors, as well as radio-guided surgery of neuroendocrine metastatic lesions, if the involved site is located in proximity to highly octreotide-avid organs such as the kidneys or spleen [46].
99mTc-EDDA/HYNIC-TOC
Being a technetium-99 m-labelled radiopharmaceutical, this molecule has definitive logistical and imaging advantages over octreoscan and MIBG considering the easy availability of the radionuclide, favorable imaging properties, early imaging time, and lesser exposure to radiation to the patients. The radionuclide 99mTc and the somatostatin analogue Tyr3-octreotide are held together by a bifunctional chelator EDDA/HYNIC, a hydrazinonicotinic acid derivative with ethylenediamine N,N’ diacetic acid (EDDA) acting as a coligand; this renders the molecule highly stable in vivo and in vitro [47]. This radiopharmaceutical has been sporadically used to evaluate solitary pulmonary nodules [48] and large cell neuroendocrine carcinoma [49] with rather disappointing results. It carries a low specificity due to high possibility of uptake in benign findings such as foreign body and suppurative inflammations, hamartomas, and tuberculomas [48].
17.8.1.2 Positron Emission Tomography (PET) Radiopharmaceuticals
18F-FDG
The utility of 18F-FDG PET scan depends principally on increased glucose metabolism and so-called metabolic trapping – greater trapping of the agent in the more metabolically active tumor cells than in surrounding tissues [50]. 18F-FDG PET/CT is one of the most commonly utilized investigations in the field of oncology today. Notwithstanding, the utility of this radiopharmaceutical is somewhat limited in the evaluation of NETs. The FDG uptake in carcinoid tumors is low, due to their low proliferative and metabolic activity and high degree of differentiation [51]. The use of 18F-FDG PET/CT may be reserved for poorly differentiated neuroendocrine cancers with little or no hormone production and a high proliferative activity (Table 17.3). Also, 18F-FDG PET/CT appears to show a higher SUVmax values for atypical carcinoids than typical carcinoids [57, 58]. This in turn leads to higher sensitivity for detection of atypical carcinoids compared to typical carcinoids. A disadvantage in using 18F-FDG is that it may be falsely positive in the inflammatory and infective lesions of lung and lymph nodes [52]. Also, other non-carcinoid tumors which can mimic bronchopulmonary carcinoid (mucoepidermoid carcinoma, adenoid cystic carcinoma, schwannoma, and inflammatory myoblastic tumors) show 18F-FDG avidity that lowers the specificity of 18F-FDG PET/CT in the detection of bronchopulmonary NETs [52, 55, 59, 60]. 18F-FDG has been rarely used in the evaluation of thymic carcinoids; a possible role of the modality may lie while searching for a cause of ectopic Cushing’s syndrome where thymic carcinoids may be identified [61–63].
Table 17.3
Studies depicting the utility of 18F-FDG PET in pulmonary carcinoids
Serial no. | Authors | Year | Methodology | Results |
|---|---|---|---|---|
1 | Erasmus et al. [51] | 1998 | Evaluated 7 cases of pulmonary carcinoids with 18F-FDG PET | Carcinoid tumors do not show high uptake on 18F-FDG PET |
2 | Bryant et al. [52] | 2006 | Evaluated 585 patients of solitary pulmonary nodule with 18F-FDG PET CT(14 cases were carcinoid tumors) | 10/14 carcinoid showed uptake on FDG PET while 4 showed no uptake. 18F-FDG PET showed increased uptake in infective lesions; lesions with SUVmax > 2.5 were more likely to be malignant |
3 | Kruger et al. [53] | 2006 | Studied 13 patients with pulmonary carcinoids (12 typical and 1 atypical). Integrated FDG PET/CT scan and surgical resection were performed in all patients | SUVmean in atypical carcinoids significantly higher than in typical carcinoids |
4 | Daniels et al. [54] | 2007 | Performed a retrospective review 18F-FDG PET of 16 patients with pathologic diagnosis of bronchial carcinoid (TC-11;AC-5) | Overall sensitivity of 75 %; higher for atypical carcinoids (80 %) than typical carcinoids (72.7 %) |
5 | Venkitaraman et al. [55] | 2014 | Prospective study on 18F-FDG PET/CT in 32 patients with clinical suspicion of BCs | The sensitivity of 18F-FDG PET was superior in patients with atypical BCs compared to those with typical BCs (100 versus 61.9 %) |
6 | Gasparri et al. [56] | 2015 | Retrospectively evaluated 97 patients with pulmonary carcinoids (65 typical, 32 atypical) who had undergone preoperative 18F-FDG PET | Overall sensitivity of 67 %; higher for atypical carcinoids (81 %) than typical carcinoids (60 %) |
68Ga-DOTA-Somatostatin Analogues (SSA)
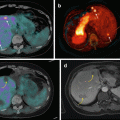
High levels of SSTR expression are generally detected in lung carcinoids, predominantly subtypes 2, 3, and 5, which has been verified by a multitude of in vitro and in vivo studies [64]. This implies that radiopharmaceuticals which target these receptors can help in the detection of these lesions. This has best been achieved with the help of 68Ga-DOTA-conjugated SSA. Three such molecules (DOTANOC, DOTATOC, DOTATATE, all labelled with 68Ga) have been developed and extensively studied in assessing their role in the detection of pulmonary carcinoids apart from neuroendocrine tumors at other sites [65, 66]. They offer distinct advantages compared to their SPECT counterparts such as octreoscan in terms of superior spatial resolution and earlier imaging times. The superior spatial resolution invariably leads to higher detection rates compared to octreoscan. They have opened up horizons in terms of understanding the biological behavior of these malignancies. The binding molecular affinities and specificities to the different somatostatin receptor subtypes vary between the three molecules [65]. Hence, although they show similar biological distribution and diagnostic accuracies, one 68Ga-DOTA-SSA molecule may show superior tumor-to-background ratios and SUVmax values compared to the other [67].
However, one feature common to the three molecules is their often documented inverse relationship compared with 18F-FDG in uptake values between typical and atypical carcinoids. Two studies at our department also prove this point satisfactorily. The study by Venkitaraman et al. [55] and Jindal et al. [59] evaluated patients with suspected bronchial carcinoids using 68Ga-DOTATOC and 18F-FDG PET/CT studies. They found that the ratio of SUVmax values of 68Ga-DOTATOC to 18F-FDG PET scans was higher in case of typical carcinoids, while atypical carcinoids showed higher SUVmax for 18F-FDG (Table 17.4).
Table 17.4
Studies depicting the utility of 68Ga-DOTA-SSA PET in pulmonary carcinoids
Serial no. | Authors | Year | Methodology | Results |
|---|---|---|---|---|
1 | Hofmann et al. [68] | 2001 | Evaluated 8 patients with histologically proven metastatic carcinoid tumors (2 bronchial, 6 abdominal) by 111In-octreotide scan and 68Ga-DOTATOC PET | 68Ga-DOTATOC PET could identify all the reference lesions, whereas 111In-octreotide imaging identified 85 % of lesions. 68Ga-DOTATOC PET identified 30 % more lesions |
2 | Kowalski et al. [69]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|