Fig. 16.1
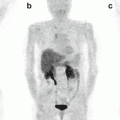
68Ga-DOTA-NOC PET/CT images of a patient with a large pNEN of the pancreatic head (a,b,c; SUVmax 40) presenting three additional focal lesions at liver level (a,d,e; SUVmax=7.4). Teaching point: the very high uptake of the lesions reflects a high expression of SSTR and a high degree of tumour differentiation that is correlated with a better prognosis
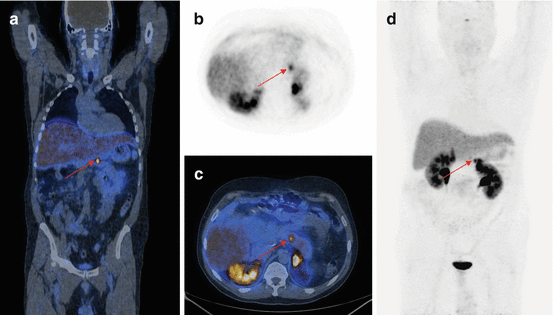
Fig. 16.2
68Ga-DOTA-NOC PET/CT coronal (a), transaxial (b, c) and MIP (d) images showing a focal area of pathologic tracer uptake at the pancreatic body (arrows) consistent with active disease localisation in a patient with MEN1 previously surgically treated for other NEN localisations (parathyroid, head of the pancreas, right liver). Teaching point: SRI PET/CT shows a high sensitivity for the detection of very small lesions
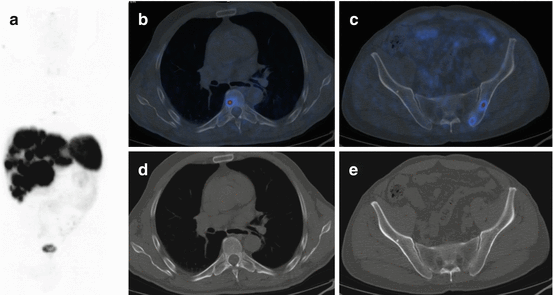
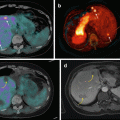
Fig. 16.3
68Ga-DOTA-NOC PET/CT image of a patient with metastatic NEN of the pancreatic tail presenting multiple liver (a; SUVmax=22) and bone (b,c,d,e; SUVmax=7) lesions. Teaching point: SRI PET/CT may document pathological lesions at bone level that do not present changes on co-registered CT
Indications to 68Ga-DOTA-peptides PET/CT [14] include staging, restaging after therapy, detection of the unknown primary tumor (in cases presenting with metastatic NEN lesions) and to assess the presence of SSTR on NET cells, in order to select patients eligible for treatment with somatostatin analogues. In fact, 68Ga-DOTA-peptide PET/CT has become an unavoidable procedure before starting target therapy with either cold or hot (PRRT, peptide receptor radionuclide therapy) somatostatin analogues. The employment of 68Ga-DOTA-peptide PET/CT as first-step examination in patients with only a clinical suspicion of NEN and the assessment of the response to therapy are on the contrary two clinical settings in which there is still debate on whether DOTA-peptides should be routinely employed.
The information derived from 68Ga-DOTA-peptide PET/CT was reported to have an impact on clinical management, especially regarding the choice of treatment (medical, PRRT, surgical) [17–19].
Finally, 68Ga-DOTA-peptide PET/CT has been reported to provide prognostic information (patients presenting lower SUVmax values were more likely to present disease progression) [20]. In fact, since 68Ga-DOTA-peptide PET/CT uptake correlates with SSTR expression on NET cells [21–23], it also provides an indirect measure of cell differentiation: lesions with a high 68Ga-DOTA-peptide uptake have a higher differentiation grade and are therefore associated with a better prognosis [23]. The SUVmax of pNEN has been reported to be significantly higher than the one of the patients with other NEN primary sites [20]. In a recent paper, specifically addressing SUVmax prognostic value in a population of 43 patients with pNEN with a long follow-up, the patients with a higher SUVmax were more likely to present stable disease at 24-month follow-up [23]. Finally, a recent report highlighted how patients with higher SUVmax were more likely to respond to treatment with either hot or cold somatostatin analogues [24].
Although SRI by means of PET/CT with 68Ga-DOTA-peptides is at present the most accurate imaging procedure to study patients with well-differentiated pNEN, it must be remembered that other tracers are more suitable for cases presenting undifferentiated forms or in cases with variable to low expression of SSTR (e.g. benign insulinoma).
16.3.1.1 Synthesis of 68Ga-DOTA-Peptides and PET/CT Imaging Protocol
The synthesis and labelling process of 68Ga-DOTA-peptides [14] is quite easy and economic: gallium can be easily eluted from a commercially available Ge-68/Ga-68 generator, and therefore there is no need of an on-site cyclotron. 68Gallium (t1/2 = 68 min) presents an 89 % positron emission and negligible gamma emission (1077 keV) of 3.2 %. The long half-life of the mother radionuclide 68Ge (270.8 days) makes it possible to use the generator for approximately 9–12 months depending upon the requirement, rendering the whole procedure relatively economic. In 2011, the EANM published guidelines for the standardisation of 68Ga-DOTA-peptide PET/CT acquisition; in particular no specific patient preparation is required, and the use of contrast media is not routinely recommended [14]. In adults, images are generally acquired 60 min after the intravenous injection of approximately 100–200 MBq of the chosen 68Ga-DOTA-peptide (TOC, NOc, TATE). Images should be reconstructed using the iterative reconstruction algorithm implemented in the system and with the system settings. Reconstruction may be performed with or without time of flight information, depending on the system capabilities.
16.3.1.2 68Ga-DOTA-Peptides and PET/CT Image Interpretation: Normal Biodistribution and Pitfalls
A 68Ga-DOTA-peptide PET/CT scan is generally reported positive when increased tracer activity is detected at sites other than the physiological biodistribution (pituitary gland, spleen, liver, adrenal glands, head of the pancreas, the thyroid, kidneys and urinary bladder).
In order to correctly interpret 68Ga-DOTA-peptide PET/CT scans, a thoughtful knowledge of the biodistribution and of common causes of false-positive and false-negative findings is mandatory.
False-positive reporting may derive from the presence of accessory spleens (rarely intra-pancreatic), splenosis, inflammation (due to the presence of SSR on activated lymphocytes and macrophages, e.g. pancreatitis) and lymphoma (e.g. primary pancreatic lymphoma). Increased tracer uptake at the head of the pancreas is a relatively frequent finding (30–60 %), not necessarily associated with the presence of disease. From a clinical point of view, the detection of a focal area of increased uptake at the head of the pancreas should be regarded more suspiciously as compared to a diffuse homogeneous uptake pattern (more likely to be benign), and further investigations should be recommended.
False-negative findings include small lesion dimension (<5 mm) and tumors with low or variable expression of SSR (e.g. benign insulinoma).
16.3.2 18F-FDG Imaging of pNEN
18F-FDG, an analogue of glucose labelled with 18F and the most commonly employed radiopharmaceutical in PET/CT studies, is not the gold standard tracer to study well-differentiated NEN due to their generally slow growing rate and a low glucose metabolism. However, undifferentiated tumors may present increased glucose consumption and therefore 18F-FDG uptake. In particular, FDG-positive sites may or may not correspond to 68Ga-DOTA-peptide uptake sites. Therefore, much attention has been recently focused on the possibility to perform double PET tracer investigation [25–27], in order to acquire both data on SSTR expression (by means of 68Ga-DOTA-peptides) and data on glucose metabolism (by means of 18F-FDG), that is well known to correlate with a more aggressive tumor behaviour [28]. In particular, with the increase in pNEN detection, as a consequence of both an improvement in lesions detection techniques and therapeutic management, there is also an increase in the number of pNEN cases presenting a more aggressive behaviour, either at first presentation or during the course of the disease. Several papers recently addressed these issues, portraying the message that 18F-FDG PET/CT may be useful to select patients with a more aggressive disease [27]. Although very interesting, the limited patient sample studied in each of the published reports (not to mention that only few specifically addressed patients with pancreatic primary), together with the lack of suggestions on how often to perform double tracer imaging during the course of the disease (that is in many cases quite long), fails to provide a definitive message to the clinicians, who still are therefore encouraged to discuss each individual case in multidisciplinary board meetings in order to decide when it is more appropriate to perform double tracer PET/CT.
Further studies are needed to better ascertain the role of double tracer imaging in clinical practise, and in particular it is mandatory to identify factors that may help to identify when a patient should be address to double tracer PET/CT imaging during the course of the disease.
16.3.3 68Ga-Exedin4 PET/CT Imaging of Pancreatic Insulinomas
Insulinomas are the most common functioning endocrine pancreatic tumors (1–2 % of all pancreatic neoplasms) [29, 30]. Their clinical presentation is characterised by hypoglycaemia secondary to insulin secretion. Delays in the diagnosis are common because the symptoms usually precede tumor detection and may be misattributed. Since aggressive surgical resection is the primary treatment option, accurate tumor localisation is important for appropriate management [29].
Insulinomas are generally solitary, confined to the pancreas and benign. However, malignant forms, although extremely rare, were reported and are characterised by an aggressive clinical behaviour, with invasion of the surrounding soft tissues/lymph nodes or distant spread (mostly to the liver) [29, 31].
To accurately image benign insulinomas, glucagon-like peptide-1 receptor (GLP-1) imaging is generally superior to SRI, due to low expression of SSTR [32, 33]. In particular the 68Ga-labelled exedin was reported to be superior to the 111-In-labelled compound [34]. However, negative GLP-1 receptor findings have been suggested to indicate the presence of a malignant insulinoma [32] that can be studied with 68Ga-DOTA-peptides [35].
16.3.4 18F-DOPA Imaging of pNEN
18F-DOPA is a metabolic tracer that can be employed to study NEN. However, due to its normal biodistribution to the pancreas [36], it is not the tracer of choice to study pancreatic tumors at primary site. 18F-DOPA may be helpful for the characterisation of well-differentiated secondary neuroendocrine lesions. However, its accuracy in well-differentiated neuroendocrine lesions detection has been reported to be inferior to 68Ga-DOTA-peptides [37, 38]. Recent reports also indicate a limited accuracy for the detection of insulinomas, although carbidopa premedication seems to improve it [39]. In this clinical setting, both 68Ga-exedin and 68Ga-DOTA-peptides seem to be more promising.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree