Fig. 13.1
Jugular PGL. Co-registered axial planes in MR TOF (time of flight) without gadolinium, CT (bone kernel), and MR TOF—CT rigid fusion with PGLs drawn in orange. 1 Jugular bulb, pars vascularis; 2 Jugular bulb, pars nervosa; 3 Hypoglossal nerve in the hypoglossal canal; 4 Facial nerve, mastoid part; 5 Mastoid cells; 6 Internal carotid artery; 7 Basilar process of occipital bone; 8 Vertebral artery (V4); 9 Occipital artery; 10 Middle meningeal artery before entering in foramen spinosum; 11 Ascending pharyngeal artery, neuromeningeal trunk; 12 Right jugular PGL (confirmed with focal uptake of 18F-FDOPA and 68Ga-DOTATATE) located in the anterior wall of jugular vein (pars vascularis of the jugular foramen); 13 Left jugular PGL extending to pars vascularis and nervosa and responsible of “wet sugar” bone erosion
Tympanic PGLs commonly arise from paraganglia adjacent to the tympanic plexus of Jacobson’s nerve on the cochlear promontory (glomus tympanicum PGLs, TP) (Fig. 13.2). They can occlude the Eustachian tube or protrude through the tympanic membrane into the external auditory canal.
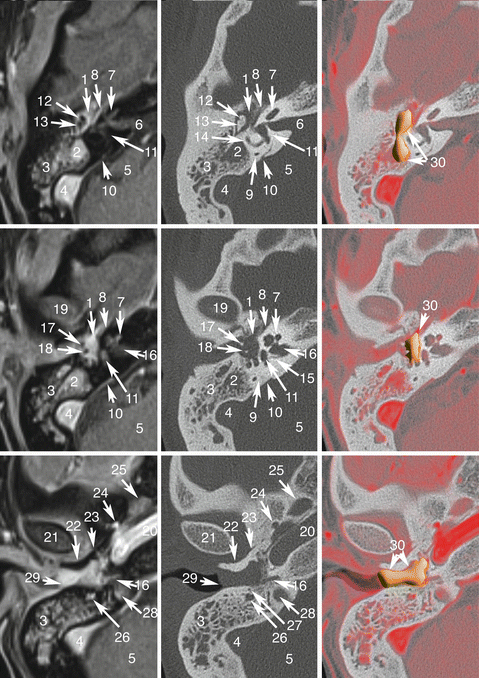

Fig. 13.2
Tympanic PGL. Co-registered axial planes in MR 3D T1 weighted with fat saturation (VIBE, Siemens) with gadolinium, CT (bone kernel), and MR T1 VIBE gadolinium—CT rigid fusion with PGLs drawn in orange. 1 Attic, filled by PGL; 2 Mastoid antrum filled by PGL; 3 Mastoid cells filled by serohemorrhagic fluid; 4 Sigmoid sinus, tumor-free and permeable; 5 Posterior fossa; 6 Internal auditory meatus opening (porus acusticus internus); 7 Cochlea; 8 Labyrinthic portion of facial nerve; 9 Posterior semicircular canal; 10 Endolymphatic sac (internal blind pouch of endolymphatic duct); 11 Vestibule, 12 Head of malleus; 13 Incus, body and short crus; 14 Lateral semicircular canal; 15 Utricularis and ampullaris posterior nerve canal; 16 Fundus of internal acoustic meatus; 17 Handle of malleus; 18 Incus, long crus; 19 Mandibular fossa of the temporal bone; 20 Carotid canal; 21 Mandibular condyle; 22 Tympanic part of temporal bone; 23 Anterior tympanosquamous fissure (Glaser’s fissure); 24 Foramen spinosum-middle meningeal artery; 25 Foramen ovale; 26 Mastoid segment of facial nerve; 27 Chorda tympani; 28 Jugular bulb (upper part); 29 Tympanic membrane (bulging); 30 Tympanic PGL
Vagal PGLs arise from paraganglia associated with the vagus nerve, most often at the level of the inferior (nodose) ganglion (glomus vagale PGL, VP) (Fig. 13.3). They may extend upward into the skull base through the jugular foramen, inferiorly into the cervical area, and laterally surrounding the internal carotid artery and lower nerves. Moreover, they can compress the pharyngeal wall.
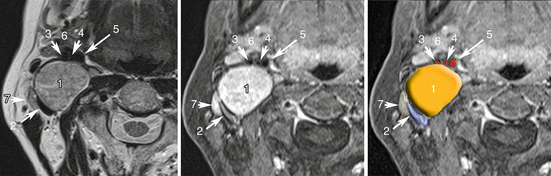

Fig. 13.3
Vagal PGL. Co-registered axial planes in MR T2 WI (a), 3D T1 weighted with fat saturation (VIBE, Siemens) with gadolinium (b). Drawn layer (c) of surrounding arteries (in red) and veins (in blue) around the PGL drawn in orange. 1 Vagal PGL, 2 Jugular vein, 3 External carotid artery, 4 Ascending pharyngeal artery, 5 Internal carotid artery, 6 submandibular gland, 7 normal node. Note that vagal nerve PGLs splay jugular veins away from the carotid arteries
Carotid PGLs arise from the carotid body at the bifurcation of the common carotid artery (glomus caroticum PGLs, CBP) (Fig. 13.4). They may extend laterally surrounding carotid vessels and lower nerves and upward into the retrostyloid parapharyngeal space.
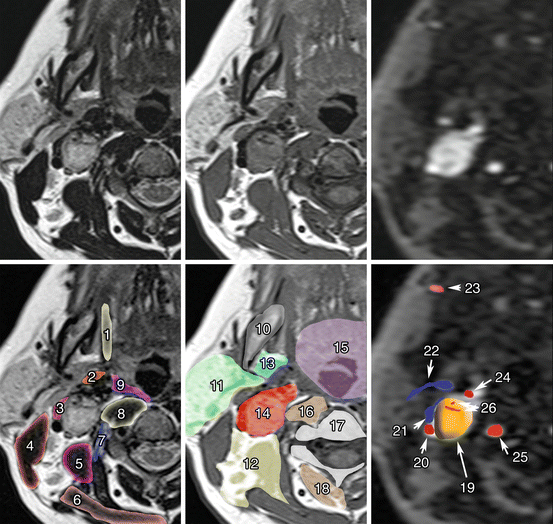

Fig. 13.4
Carotid body PGL. Co-registered axial planes in MR T2 WI (a), MR T1 WI (b), and time-resolved 4D MRA at an early arterial phase of gadolinium injection (c). Drawn layers of surrounding muscle (d), deep spaces of the neck (e), and vascular structures (f) around the carotid PGL. 1 Styloglossus muscles; 2 Stylopharyngeus muscles; 3 Digastric muscle, posterior belly; 4 Sternocleidomastoid muscle; 5 Scalene muscles; 6 Trapezius muscle; 7 Longus capitis muscle; 8 Longus colli muscle; 9 Middle pharyngeal constrictor muscle; 10 Mandible; 11 Parotid space; 12 Posterior triangle of the neck; 13 Parapharyngeal space, prestyloid; 14 Parapharyngeal space, post-styloid (vascular space); 15 Mucosal space (visceral); 16 Retropharyngeal and revertebral spaces; 17 Vertebrae; 18 Paravertebral space; 19 Carotid body PGL; 20 Internal carotid artery; 21 Jugular vein; 22 Retromandibular vein; 23 Facial artery; 24 External carotid artery; 25 Vertebral artery; 26 Internal arterial feeders (flow voids). Note that carotid body PGL (glomus) splays external carotid away from the internal carotid artery
PGLs may also develop in the anterior and middle mediastinum. They have the same embryologic origin as HNPGLs, and are therefore considered within the same entity.
Unusual or exceptional locations have also been reported, such as PGLs of the larynx, thyroid, sinonasal region, nasopharynx, orbit, and tongue. Exceptional cases of PGLs of the cervical sympathetic chain have been reported.
13.4.2 Typical Imaging Finding on CT and/or MRI
HNPGLs usually demonstrate marked enhancement of intra-tumoral vessels following contrast administration on CT, a low signal on T1-weighted images, and an intermediate to high signal on T2-weighted MRI images. They also often enhance intensely after gadolinium injection on MRI. Flow signal voids in the tumor are typical of PGL, with a “salt and pepper” appearance on spin-echo sequences. These flow voids correspond to intra-tumoral arterial vessels on MR angiography [15–19]. These MR angiography sequences can be achieved without gadolinium (3D time of flight) or with gadolinium injection (3D time-resolved gadolinium). These sequences have been shown to be highly informative, with sensitivities and specificities of 90 % and 94 % [16] and 100 % and 94 % [17], respectively. There is a recent trend of using time-resolved 4D gadolinium MR angiography [19]. The 4D time-resolved imaging of contrast kinetics (TRICKS) technique enables evaluation of both intra-tumoral vessels (early arterial phase) and tumor perfusion, including capillary permeability. Fusion images between 3D volumetric interpolated fat-saturated (FATSAT) T1-weighted (VIBE) and 4D MR angiography are particularly informative. Diffusion-weighted imaging (DWI), which is dependent on tissue cellularity, may be useful in preoperative characterization and prognosis assessment of PGL, but further evaluation is needed [20].
13.4.3 Tumor Uptake Using Specific Radiopharmaceuticals
Radionuclide imaging techniques now play a crucial role in the evaluation of HNPGLs. Several excellent studies have demonstrated the superiority of Octreoscan for HNPGLs compared to metaiodobenzylguanidine (123/131I-MIBG), with sensitivities of 89–100 % and 18–50 %, respectively [21–26]. However, the sensitivity of this imaging modality needs to be revised downward, because some lesions are only a few millimeters in size, and are therefore not detectable by available cameras [27]. The recently introduced hybrid SPECT/CT cameras have increased diagnostic confidence in image interpretation and enhanced sensitivity, but practical constraints such as long imaging times remain important limitations. PET imaging is a highly sensitive functional imaging technique for HNPGLs.
13.4.3.1 18F-Fluorodihydroxyphenylalanine
18F-fluorodihydroxyphenylalanine (18F-fluorodopa, 18F-FDOPA) is taken up through neutral amino acid transporters (mainly LAT1/LAT2), decarboxylated into 18F-fluorodopamine by aromatic L-amino acid decarboxylase, and concentrated in intracellular vesicles. 18F-FDOPA PET/CT was found to be a highly sensitive (>90 % for HNPGLs) and specific (95–100 %) imaging modality for PGL detection, especially for HNPGLs [28–32]. 18F-FDOPA PET was also found to be superior to octreoscan [28, 30] in the detection of HNPGLs, mainly due to increased sensitivity to PET cameras. However, 18F-FDOPA is not routinely available at most imaging centers worldwide.
13.4.3.2 18F-Fluorodopamine
18F-fluorodopamine (18F-FDA), which uses the cellular norepinephrine transporter system expressed on PGL cells, is not recommended for the evaluation of these tumors due to its suboptimal sensitivity.
13.4.3.3 68Ga-Labeled Somatostatin Analogues
More recently, PET/CT imaging with 68Ga-labeled somatostatin analogues (68Ga-DOTA-SSAs) has rapidly evolved, since it does not require a cyclotron to make the radiotracer. All 68Ga-DOTA-SSAs (DOTATOC, DOTATATE, and DOTANOC) effectively target somatostatin receptor subtype 2 (SST2) (IC50: 2.5 nM, 0.2 nM, and 1.9 nM, respectively), which is the most overexpressed subtype in PGLs. The low expression of SST5 observed in PGLs constitutes a major difference with GEP-NETs.
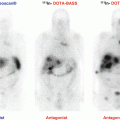
Moreover, a special advantage of labeled SSAs is that, unlike 18F-FDOPA, they can be used in the radioactive treatment of these tumors (as theranostic agents). In 2014, 68Ga-DOTATATE was approved with an orphan drug status by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in the imaging of gastroenteropancreatic neuroendocrine tumors. The use of 68Ga-DOTA-SSA in the context of PHEO/PGLs has been less studied but has shown excellent preliminary results in localizing these tumors [33–38]. Head-to-head comparison between 68Ga-DOTA-SSA and 18F-FDOPA PET has been performed in only four studies: one retrospective study from Innsbruck Medical University (68Ga-DOTATOC in 20 patients with unknown genetic background) [39], two prospective studies from the NIH (68Ga-DOTATATE in 17 and 20 patients) [40–42], and one prospective study from La Timone University Hospital (68Ga-DOTATATE in 30 patients) [43]. In these studies, 68Ga-DOTA-SSA PET/CT detected more primary head and neck PGLs as well as SDHx-associated PGLs than 18F-FDOPA PET/CT. The performances of the different imaging techniques are detailed in Table 13.1. In the context of sporadic cases, 68Ga-DOTATATE and 18F-FDOPA PET/CT have similar sensitivities with higher uptake values and metabolic tumor volumes with 68Ga-DOTATATE compared to 18F-FDOPA (Fig. 13.5).
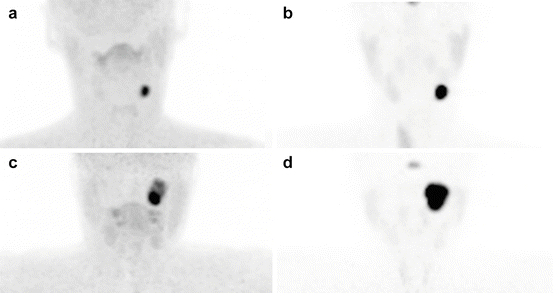
Table 13.1
Comparison of different imaging techniques for the localization and staging of HNPGLs
Sensitivity | Specificity | Locoregional staging (delineation) | Malignancy prediction | Advantages and drawbacks | Mean estimated effective dose equivalent | |
|---|---|---|---|---|---|---|
CT | 80–90 % | 90 % | High | Unreliablea | High spatial resolution Low-motion artifacts Accurately delineates temporal bone extension | 3 mSv for unenhanced CT and 5 mSv for CT angiogram |
MR | 80–90 % | 90 % | High | Unreliablea | No iodinated media No radiation exposure Accurately delineates soft tissue extension | None |
PET/CT 18F-FDOPA | 90 % | >95 % | Low | Unreliablea | Decreased sensitivity in SDHx-related PGL (especially sympathetic ones) Very high sensitivity for PHEO detection | 0.025 mSv/MBq for 18F-FDOPA and 3 mSv for low-dose CT |
PET/CT 18F-FDG | 60–70 % | 80–90 % | Low | Unreliablea | Increased sensitivity in SDHx tumorsb Accurately detects concomitant abdominal PGLs that can be missed by 18F-FDOPA | 0.019 mSv/Mbq for 18F-FDG and 3 mSv for low-dose CT |
PET/CT 68Ga-DOTATATE | 100 % | 90 % | Low | Unreliablea | Highly sensitive in HNPGLs, regardless of genotype High TBR Extemporaneously prepared | 0.021 mSv/Mbq for 68Ga-DOTATATE and 3 mSv for low-dose CT |

Fig. 13.5
Examples of 18F-FDOPA and 68Ga-DOTATATE tumor uptake patterns in sporadic HNPGLs. (a, b) Left CBP, (c, d) left jugular PGL. (a, c) 18F-FDOPA PET/CT (MIP image), (b, d) 18F-FDG PET/CT (MIP image)
It should be noted that SST-based imaging may be somewhat less specific than 18F-FDOPA in the evaluation of these tumors and could be falsely positive in metastatic lymph nodes due to various cancers; meningiomas; other central nervous, inflammatory processes; and some rare conditions such as fibrous dysplasia [43]. Nevertheless, this is often not a serious issue, since HNPGLs have a specific location and exhibit highly elevated uptake values [38].
13.4.4 Presence of Germline Mutation in HNPGL Susceptibility Genes and/or Family History of PGL/PHEOs
HNPGLs can be sporadic or can occur as components of hereditary syndromes in up to 30–40 % with variable penetrance and increased risk for recurrent behavior and tumor multiplicity. Of all the known genetic mutations, mutations in SDHD are currently the leading cause of hereditary HNPGLs (>50 %), followed by SDHB (20–35 %) and SDHC (15 %) mutations [44–46]. Some correlations between the gene(s) involved and tumor location have been found. SDHD-linked patients have a 75 % risk of developing HNPGLs throughout their life, with concomitant thoracoabdominal PGLs in about 10 % of cases [27]. SDHB-linked PGL syndrome is characterized by a high rate of retroperitoneal PGL or HNPGLs, but a combination of sympathetic and parasympathetic PGLs is found less frequently [27]. Furthermore, it should be noted that multiple PGLs at a very young age found in several family members may be related to SDHAF2 mutations [47]. So far, only HNPGLs have been reported in these rare families. The inheritance pattern of the SDHB and SDHC genes is autosomal dominant, whereas for SDHD and SDHAF2 genes, the disease occurs only when the mutations are inherited from the father, which is consistent with maternal imprinting [48]. Until present, mutations in SDHA have been described in several isolated cases. Major predictors for hereditary HNPGLs are a family history of PGL (especially those related to SDHD mutations), a previous personal history of HNPGLs or sympathetic PGL, and multifocality or a characteristic syndromic presentation [5, 46]. Currently, a well-characterized syndromic presentation includes the existence of other tumor types associated with the presence of a PGL (e.g., renal cell carcinoma, gastrointestinal stromal tumor, and pituitary adenomas can also be related to SDHx mutations) [49, 50].
Imaging phenotypes may vary across genotypes. Although 18F-FDG is not specific, this imaging modality has clearly shown a higher sensitivity over CT/MRI, 123/131I-MIBG, 18F-FDA, and 18F-FDOPA [32, 51, 52]. More recently, 68Ga-DOTATATE PET/CT detected more SDHx-associated PGLs than 18F-FDOPA PET/CT [39–41, 43]. It is expected that 68Ga-DOTA-SSA PET/CT will become the preferred functional imaging modality for HNPGLs in the near future.
13.4.5 Differential Diagnosis
There are several potential causes to consider in the differential diagnosis (Tables 13.2 and 13.3).
Table 13.2
Classification of HNPGLs and their main clinical and radiological differential diagnoses
Type | Differential diagnoses |
|---|---|
Jugular PGL | Schwannoma Neurofibroma Meningioma Carcinoma (primary and metastatic) and tumor extension from nasopharyngeal carcinoma Angiosarcoma and chondrosarcoma Plasmacytoma, hemangiopericytoma Endolymphatic sac tumor Miscellaneous lesions (i.e., internal jugular vein thrombosis, Langerhans cell histiocytosis) |
Tympanic PGL | Middle ear adenoma (carcinoid) Meningioma Hemangioma of geniculate ganglion Schwannoma Neurofibroma Miscellaneous lesions (i.e., aberrant internal carotid artery, mega bulb jugular, dehiscent jugular bulb, Langerhans cell histiocytosis) |
Vagal PGL | Schwannoma Neurofibroma Lymph node metastasis (nasopharynx/oropharynx, thyroid cancer) Lymphoma Ganglioneuroma Hemangiopericytoma Miscellaneous lesions (i.e., abscess, internal carotid artery aneurysm, internal jugular vein thrombosis, vascular malposition, organizing hematoma) |
Carotid body PGL | Carotid artery aneurysm Vasculitis of the carotid artery Lymphadenopathy Schwannoma Neurofibroma Branchial cleft cyst Accessory thyroid gland |
Table 13.3
Differential diagnosis of post-styloid retropharyngeal masses: imaging features on MRI and molecular imaging
Paraganglioma | Nodal metastasis | Schwannoma | Neurofibroma | |
|---|---|---|---|---|
MRI | Numerous arterial feeders Spin echo: flow void (“salt and pepper” appearance on T1- and T2-weighted images) TOF: hyperintense vessels within the lesion 3D (or 4D) gadolinium angiograms: avid enhancement | Variable appearance Well-defined margins Moderate signal in T2 Restricted diffusion Heterogeneous enhancement usual | Well-defined margins Moderate to high signal intensity on T2 No restriction of diffusion Heterogeneous enhancement seen in large tumors with degenerative changes (cystic, pseudocystic) | IDEM schwannoma Central low signal on T2 may be seen (“target sign”) |
PET/CT 18F-FDG | Moderate to high uptake (highly elevated in SDHx) | Low to high uptake depending on the primary tumor | Low to high uptake (even in benign cases) | Low uptake (high uptake values in malignant forms) |
PET/CT 18F-FDOPA | High uptake | No significant uptake | No significant uptake | No significant uptake |
PET/CT 68Ga-SSA | High uptake | No significant uptake (possible high uptake in nodal metastases from thyroid cancers or nasopharyngeal cancers) | No significant uptake | No significant uptake |
13.5 Role of Imaging and Clinical Impact
The aim of pre-therapeutic imaging is to provide the most complete staging of the disease. In this way, treatment can be tailored to each specific situation. The initial staging of a HNPGL is currently based on the use of anatomical and functional imaging approaches in order to determine the tumor extension into the bone and surrounding soft tissue; to determine tumor multiplicity, not only in the head and neck area but elsewhere in the body; and finally to exclude any metastases.
13.5.1 Detection of Multifocal/Metastatic Disease
To determine whether additional HNPGLs are present, MRI is inferior to 18F-FDOPA PET/CT and 68Ga-DOTA-SSA. Therefore, it is currently recommended that all patients with HNPGLs, especially SDHx mutation carriers, be assessed by 68Ga-DOTA-SSA PET/CT. Although both functional imaging modalities are relatively new and more studies are needed, the published results are strongly supportive [33–35, 39, 41, 43, 53] (Figs. 13.6 and 13.7). In absence of available 68Ga-DOTA-SSA, a combination of 18F-FDOPA and 18F-FDG PET/CT is recommended, especially for patients with multifocal tumors [29, 54] (Fig. 13.8). One of the main drawbacks of 68Ga-DOTA-SSA is the very high physiological uptake by healthy adrenal glands [55]. This could be a critical problem for detecting small PHEOs in hereditary syndromes.
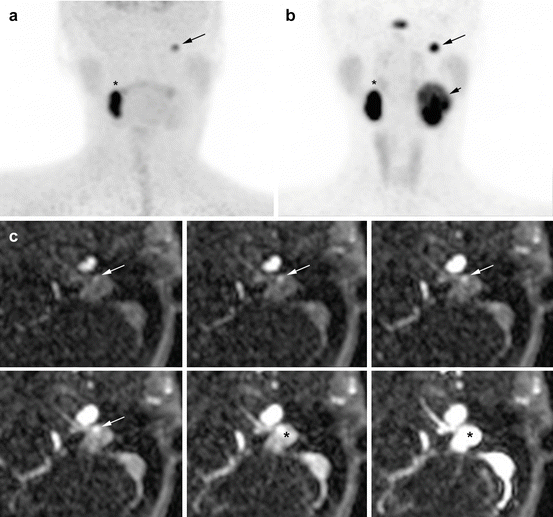
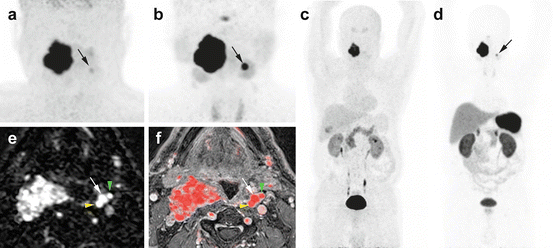
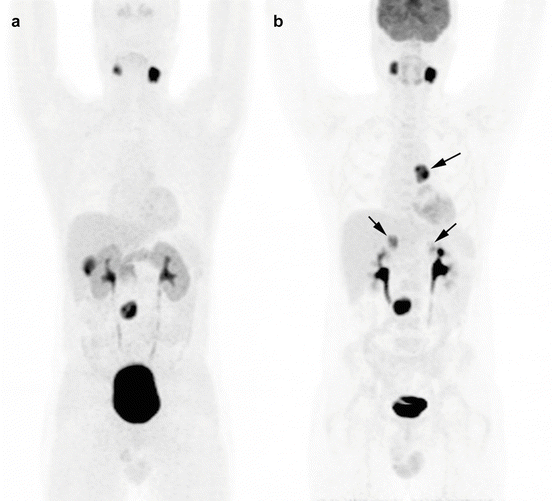

Fig. 13.6
Superiority of 68Ga-DOTATATE to 18F-FDOPA PET/CT and MRI in an SDHD patient. (a) 18F-FDOPA PET (MIP) missed the large left VP (long arrow). (b) 68Ga-DOTATATE (maximal intensity projection (MIP)) showing three PGLs (right VP = asterisk, left VP = small arrow, left JP = long arrow). (c) Contrast-enhanced time-resolved 4D MRA at 3 tesla showing a small nodule located in the wall of the jugular bulb with an early arterial enhancement (white arrow), prior to jugular vein and sigmoid sinus lumen contrast media injection filling (*). This lesion was detected only upon knowledge of the PET results

Fig. 13.7
Superiority of 68Ga-DOTATATE to 18F-FDOPA PET/CT and MRI in an SDHD patient. Multicentric SDHD-related PGL syndrome. (a) Craniocervical 18F-FDOPA PET image (MIP) showing the two lesions but was slightly positive on the left side (arrow). (b) Craniocervical 68Ga-DOTATATE image (MIP) showing two VP. (c) WB 18F-FDOPA PET (MIP) which failed to demonstrate the left VP. (d) WB 68Ga-DOTATATE (MIP) image showing the known two lesions. (e) Early arterial phase of contrast-enhanced time-resolved 4D MRA at 3 tesla. (f) 4D MRA fused with 3D gadolinium-enhanced sequences T1 fat-saturated post-gadolinium (3D VIBE) image. Both images show the early arterial enhancement pattern of the two VPs (white arrows showing the left VP). Note the discrete splaying between internal carotid artery (yellow arrowhead) and external carotid artery (green arrowhead)

Fig. 13.8
Superiority of 18F-FDG PET/CT to 18F-FDOPA PET/CT in the detection of additional PGL of sympathetic origin in an SDHD patient. (a) 18F-FDOPA PET/CT (MIP image), (b) 18F-FDG PET/CT (MIP image). Arrows mark the false-negative 18F-FDOPA tumors
Although rare, malignancy often requires dramatically different patient management. When metastases are present, the goal of imaging is to precisely assess the anatomy of these tumors in order to carefully select patients who could benefit from aggressive but complete surgical resection with the intent to cure as opposed to “debulking” strategies [56]. To this end, 68Ga-DOTA-SSA is superior to 18F-FDG PET/CT and 18F-FDOPA PET/CT in SDHB patients [40] (Figs. 13.9 and 13.10).
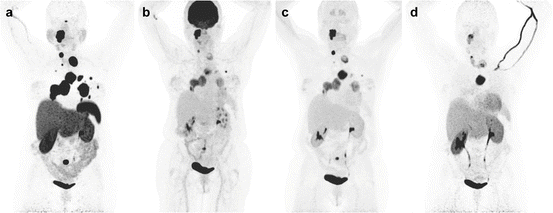
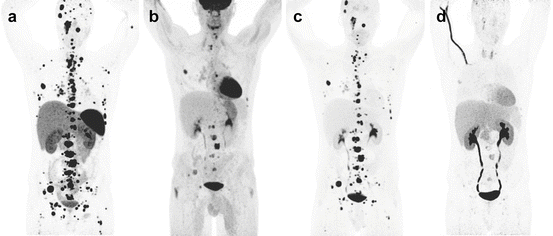

Fig. 13.9
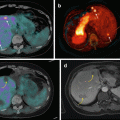
Superiority of 68Ga-DOTATATE to other molecular imaging modalities in SDHB-related metastatic HNPGL. Imaging of metastatic head and neck PGL with 68Ga-DOTATATE (a), 18F-FDG (b), 18F-FDOPA (c), and 18F-FDA PET/CT (d) in a 63-year-old female patient with SDHB mutation. First diagnosed with CB tumor on the right side at the age of 27. Recurrence of CB tumor as well as glomus vagale tumor and diagnosis of metastatic disease to the neck, mediastinum, lungs, and bones at the age of 49

Fig. 13.10
Superiority of 68Ga-DOTATATE to other molecular imaging modalities in SDHB-related metastatic HNPGL. Imaging of metastatic head and neck PGL with 68Ga-DOTATATE (a), 18F-FDG (b), 18F-FDOPA (c), and 18F-FDA PET/CT (d) in a 36-year-old male patient with SDHB mutation, first diagnosed with a paraganglioma of the right foramen jugulare at the age of 19 with partial resection. First diagnosis of widespread bone metastatic disease in 2012
13.5.2 Assessment of Locoregional Extension
Anatomic imaging serves as the first-line modality in the locoregional staging of these tumors. CT offers several advantages over MRI (e.g., better spatial resolution and less motion artifacts) and enables better evaluation of the temporal bone extension of JP and TP. MRI provides better soft tissue contrast than CT, and thus, it offers unique information for tumor delineation. MRI is also preferred if radiotherapy or stereotactic radiosurgery (SRS) is considered. Several classifications (i.e., Fisch and Mattox’s or Glasscock and Jackson’s for TP and JP, Netterville’s for VP, Shamblin’s for CBP) help predict surgical outcome and should be used in the evaluation of these patients [57–60] (Table 13.4, Figs. 13.11 and 13.12).
Table 13.4




Classifications of HNPGLs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree