First syndromic manifestation
Context at PPGL presentation (in index cases)
PHEO at presentation
Additional extra-adrenal PGL
Predominant secretion
PPGL-associated malignancy risk
MEN2
MTC
Adult
Possible phenotypic features of MEN2
Frequent family history of MTC/PHEO/PGL
Uni- or bilateral
Rare
EPI
Very low
NF1
Neurofibromas
Adult
Phenotypic feature of NF1
Possible family history of NF1
Often unilateral
Rare
EPI
Very low
TMEM127
PHEO
Adult
Possible family history of PHEO
Uni- or bilateral
No
EPI
Low
MAX
PHEO
Young adult
Frequent family history of PHEO/PGL
Bilateral
Possible
NE
Moderate
VHL
PHEO/PGL
Young adult
Frequent family history of PHEO/PGL
Uni- or bilateral
Frequent
NE
Low
SDHB
PHEO/PGL
Adult
Possible family history of PHEO/PGL
Often unilateral
Frequent
NE and/or DA
High
SDHD
PHEO/PGL
Adult
Frequent family history of PHEO/PGL
Uni- or bilateral
Frequent
NE and/or DA
Moderate
SDHC
PHEO/PGL
Adult
Possible family history of PPGL
Rare
Frequent
NE
Low
HIF2A
Congenital polycythemia
Adolescent-young adult
Female
Absence of family history of PHEO/PGL
Very rare
Almost constant
NE
Moderate
14.5 Spectrum of Hereditary Syndromes and Phenotype-Genotype Correlations
Research in molecular genetics has resulted in the identification of more than 20 susceptibility genes for tumors of the entire paraganglia system [7, 25]. Most PHEOs occur sporadically, whereas the majority of symp-PGLs are associated with germline driver mutations. Depending on their location, the most commonly found gene mutations are (1) unilateral PHEO: succinate dehydrogenase complex subunit B or D (SDHB or SDHD) and VHL; (2) bilateral PHEO: SDHB, RET, VHL, NF1, MYC-associated factor X (MAX), and TMEM127; and (3) symp-PGLs with or without PHEO: SDHB, SDHD, VHL, and HIF2A. Other genes account for a small minority of cases. Recent tumor sequencing has also led to the identification of somatic events in a large number of PHEOs/PGLs (The Cancer Genome Atlas, unpublished observations) (Table 14.1). Patients presenting with metastatic disease mainly include those with SDHB and perhaps SDHD (excluding HNPGLs), FH, and MAX-related PHEOs/PGLs, although, at present, about 50 % of metastatic PHEOs/PGLs are non-hereditary [5].
14.6 Differential Diagnosis
There are several potential causes to consider for the differential diagnosis in the presence of an adrenal or extra-adrenal mass (Table 14.2). However, the masses that belong to either PHEO or PGL are usually detected by imaging-specific characteristics that include the value of Hounsfield units (HU), T2-weighted bright images, and positivity on PHEO-/PGL-specific functional imaging, as described below.
Table 14.2
Main causes of solid extrarenal retroperitoneal masses
Localization | Cause |
|---|---|
Adrenal masses | Adrenocortical adenoma Adrenocortical carcinoma (ACC) Pheochromocytoma Adrenocortical hyperplasia Lymphoma |
Metastasis Myelolipoma Angiomyolipoma Ganglioneuroma Hematoma (may coexist with tumors, especially PHEO) Oncocytoma Granulomatous inflammation Sarcoma | |
Extra-adrenal retroperitoneal masses | Neurogenic tumor (schwannoma, neurofibroma) Ganglioneuroma Paraganglioma Lymph node (malignancies, inflammatory origin, Castleman disease) Gastrointestinal stromal tumor (GIST) Sarcoma (liposarcoma, leiomyosarcoma, other) Solitary fibrous tumor |
14.7 Typical PHEO/PGL Imaging Finding on CT and/or MRI
On non-contrast computed tomography (CT), PHEO/PGL can demonstrate a variety of appearances. Two thirds of PHEO/PGLs are solid, while the remainder are complex or have undergone cystic or necrotic changes [26]. Typically, the CT attenuation of PHEO/PGL is about soft tissue attenuation and thus greater than 10 HU, with most PHEOs/PGLs 20–30 HU or higher. PHEO/PGL can present with a high attenuation due to the presence of hemorrhage or calcifications. In contrast, necrotic tissue presents with a low attenuation. Typically, a PHEO/PGL demonstrates avid enhancement (often greater than 30 HU) [27]. In addition, enhancement can be heterogeneous, or there may be no enhancement due to cystic, necrotic, or degenerated regions within the lesion [28]. On magnetic resonance imaging (MRI), the classic imaging appearance of PHEO/PGL is “light bulb” bright on T2-weighted imaging. In reality, 30 % of PHEOs/PGLs demonstrate moderate or low T2-weighted signal intensity [27, 29]. PHEOs/PGLs typically demonstrate avid contrast enhancement following the administration of intravenous gadolinium-based contrast material [30, 31].
14.8 Typical Imaging Finding on Molecular Imaging
Nowadays, positron emission tomography (PET) is a cornerstone in the evaluation of hereditary as well as non-hereditary PHEOs/PGLs. The broad diversity of PET biomarkers enables assessment of different metabolic pathways and receptors. Beyond its localization value, this imaging modality provides unique opportunities for better characterizing these tumors at molecular levels (e.g., the presence of catecholamines and their metabolites, specific cell membrane receptors and transporters), mirroring ex vivo histological classification but on a whole-body, in vivo, scale (Table 14.4).
Table 14.4
Comparison of different PET radiopharmaceuticals in the detection of metastases PHEO/PGL (number of sites) (40, 42Timmers, 49)
Tracer | Molecular target | Cellular retention | Specificity (%) | Sensitivity sporadic | Sensitivity SDHx |
|---|---|---|---|---|---|
18F-FDA | Norepinephrine transporter | Neurosecretory vesicles | 100 | 78 | 52 |
18F-FDOPA | Neutral amino acid transporter system L (LATs) | Decarboxylation (AADC) | >95 % | 75 | 61 |
68Ga-SSA | Somatostatin receptors | Internalization (agonists) | 90 % | 98 | 99 |
18F-FDG | Glucose transporters (GLUTs) | Decarboxylation (hexokinase) | 80 % | 49 | 86 |
Thus, successful PHEO-/PGL-specific localization depends on the presence of molecules (imaging targets) for which PET radiopharmaceuticals are currently available. Based on several recent studies, it has been uncovered that PHEO-/PGL-specific imaging targets have various expressions based on whether these tumors belong to pseudohypoxic or kinase signaling clusters, present as metastatic, are located in or outside the adrenal gland, or are derived from the sympathetic or parasympathetic nervous system. Currently, 18F-fluorodeoxyglucose (18F-FDG) is the most accessible PET radiopharmaceutical, but lacks specificity for these tumors. 18F-fluorodopamine (18F-FDA) and 11C-hydroxyephedrine (11C-HED) are the most specific tracers for chromaffin tumors, but are available in very few centers and fail in metastatic and hereditary PHEOs/PGLs [32–34].18F-fluorodyhidroxyphenylalanine (18F-FDOPA) is available from different pharmaceutical suppliers, but its sensitivity widely depends on the genetic background and whether the PHEO/PGL is sympathetic or parasympathetic [35–37]. Metastatic behavior of these tumors can also affect the expression of amino acid transporters [38]. Newly developed 68Ga-labeled peptides, as in other neuroendocrine tumors, have shown very interesting results and, in our opinion, should be positioned first for many indications due to their exceptional affinity to somatostatin receptor type 2 found on these tumors [39–44].
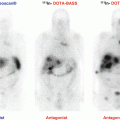
PHEOs and PGLs usually have highly elevated uptake values with specific radiopharmaceuticals based on their genetic background. For example, 18F-FDOPA PET/CT was replaced by 68Ga-DOTATATE PET/CT as the best available imaging modality for metastatic PHEO/PGL, especially in those with SDHB mutations and head and neck PGLs [40–42]. The Octreoscan has been suggested not to be used anymore due to its suboptimal performance in the detection of these tumors and the growing availability of 68Ga-DOTATATE PET/CT. However, the use of other 68Ga-labeled DOTA analogues (68Ga-DOTATOC and 68Ga-DOTANOC) needs to be confirmed in a large population of patients. A variety of new radiopharmaceuticals have been developed as potential competitors of 68Ga-DOTATATE (68Ga-labeled somatostatin antagonists, 64Cu-labeled SSA [45], or 18F-SiFAlin (silicon-fluoride acceptor)-modified TATE), but they need to be evaluated [46].
14.9 Relationship Between Genotype and Imaging Phenotype
Proper evaluation of PHEO/PGL is a key point for choosing the necessary treatment plan for follow-up and outcome for these patients. The presence of SDHx mutations markedly influences sensitivity of 18F-FDG, 18F-FDOPA, and 68Ga-DOTA-SSAs PET/CT. 18F-DOPA PET/CT has a sensitivity approaching 100 % for sporadic PHEO and a very high specificity (95 %), but can miss tumors in SDHx-mutated patients. 18F-FDOPA PET/CT still remains a very good modality for the detection of some metastatic PHEO/PGLs – it ranks as the second best for the detection of HNPGLs, and it can also be used for patients with non-hereditary metastatic PHEO/PGL [18, 35, 36, 38, 47, 48]. By contrast, SDHx tumors usually exhibit highly elevated 18F-FDG uptake values. However, 18F-FDG PET/CT positivity is present in about 80 % of primary PHEOs. Thus, 18F-FDG PET/CT remains a good alternative for the detection of metastatic PHEO/PGL, especially those related to SDHx mutations [49]. Several potential diagnoses should be considered in cases of 18F-FDG-avid adrenal masses (Table 14.3).
Table 14.3
Differential diagnosis of highly 18F-FDG-avid adrenal masses (adrenal to liver SUVmax ratio >3, A/L >3)
Tumor type | Typical feature on 18F-FDG PET/CT | Major criteria for diagnosis |
|---|---|---|
PHEO | Well-circumscribed mass with a heterogeneous uptake Moderate to high avidity Central area of low or absent avidity BAT uptake (periadrenal) | Elevated metanephrines Family history PHEO/PGL predisposing mutation Multifocality |
Adrenocortical carcinoma | Often irregular mass with a heterogeneous uptake Moderate to high avidity Often more rapid growth | Elevated steroid secretion Venous tumor extension (vena cava) Liver/lung metastases |
Lymphoma | Poorly circumscribed mass with a homogeneous uptake Highly elevated A/L (often >8) | Bilateral adrenal and lymph node involvement, elevated serum lactate dehydrogenase (LDH) |
Adrenal oncocytoma | Well circumscribed with a homogeneous uptake Highly elevated A/B (often >8) | Possible elevation of cortisol or androgens |
Metastases | Variable features Moderate to high avidity | Personal history of cancer Often extra-adrenal metastases |
The use of 68Ga-DOTA-SSAs in the context of PHEOs/PGLs has been studied less, but has shown excellent preliminary results in localizing these tumors, especially metastatic and head and neck ones, as discussed above. A head-to-head comparison between 68Ga-DOTA-SSA and 18F-FDOPA PET has been performed in only five studies: one retrospective study from Innsbruck Medical University (68Ga-DOTATOC in 20 patients with unknown genetic background) [50], three prospective studies from the NIH (SDHB, HNPGL, and sporadic metastatic PHEOs/PGLs) (68Ga-DOTATATE in 17 and 20 patients), and one prospective study from La Timone University Hospital (68Ga-DOTATATE in 30 patients) [40–42]. In these studies, 68Ga-DOTA-SSA PET/CT detected more primary head and neck PGLs as well as SDHx-associated PGLs than 18F-FDOPA PET/CT [51]. By contrast, in the context of sporadic PHEO, 18F-FDOPA PET/CT may detect more lesions than 68Ga-DOTATATE, although larger studies are needed to confirm those results [51]. One of the main drawbacks of 68Ga-DOTA-SSA is the very high physiological uptake by healthy adrenal glands [52]. Furthermore, there are also different affinities of various DOTA-SSAs to somatostatin receptors. DOTATATE has the best affinity to somatostatin receptor type 2, mostly expressed on PHEO/PGLs, followed by DOTATOC. DOTANOC has the lowest affinity to somatostatin receptor type 2 and has some affinity to somatostatin receptor type 5, which is least abundant on PHEOs/PGLs. Therefore, the use of DOTANOC may result in suboptimal detection of PHEO/PGL, especially their metastatic lesions. Studies comparing 68Ga-DOTATATE and 68Ga-DOTATOC are currently unavailable. Excellent results with DOTA analogues in both sporadic as well as SDHx-related metastatic PHEOs/PGLs resulted in the use of 177Lu-DOTATATE (Lutathera) in radiotherapy of these tumors [53–57]. This is followed by the preparation of clinical protocols in order to properly assess the efficacy of this treatment on a large population of well-characterized patients with metastatic or inoperable PHEO/PGL (Lin, Pacak et al. NIH protocol in preparation, 2016).
14.10 Role of Radionuclide Imaging
Successful PHEO/PGL management requires an interdisciplinary team approach. Precise identification of clinical context and the genetic status of a patient enables a personalized use of functional imaging modalities. Currently, it is recommended to adopt a tailored approach using a diagnostic algorithm based on tumor location, biochemical phenotype, and any known genetic background (Table 14.5) [48, 58].
Table 14.5
Stepwise molecular imaging approaches for PHEO/PGL
Location | Other related tumor conditions | First line | Second line | |
|---|---|---|---|---|
MEN2 | Adrenal | MTC, parathyroid adenoma, or hyperplasia | 18F-FDOPA | 123I-MIBG |
NF1 | Adrenal | Neurofibromas, MPNSTs, and gliomas | 18F-FDOPA | 123I-MIBG |
TMEM127 | Adrenal | RCCs | 18F-FDOPA | 123I-MIBG |
MAX | Adrenal | None reported | 18F-FDOPA | 123I-MIBG |
VHL | PHEO/PGL | RCC and CNS Hemangioblastomas | 18F-FDOPA | 68Ga-DOTATATE |
SDHB | PHEO/PGL | GISTs and RCCs Pituitary adenoma | 68Ga-DOTATATE | 18F-FDG |
SDHD | PHEO/PGL | GIST, RCC, and pituitary adenoma | 68Ga-DOTATATE | 18F-FDG |
SDHC | PHEO/PGL | GIST | 68Ga-DOTATATE | 18F-FDG |
HIF2A | PHEO/PGL | Somatostatinomas | 18F-FDOPA | 18F-FDA |
14.10.1 Diagnosis of PHEO or Symp-PGL
14.10.1.1 Adrenal Mass
Functional imaging should be used in a minority of cases, such as those with suspicion of nonfunctioning PHEO on CT/MRI, elevation of plasma or urine normetanephrine in the presence of an adrenal mass, acute cardiovascular complication in the critical care setting together with the presence of an adrenal mass, hemorrhagic adrenal masses, and either elevated plasma metanephrine or normetanephrine in renal insufficiency. Elevation of metanephrine in the plasma or urine in the presence of an adrenal mass does not call for the use of functional imaging since metanephrine is 99 % derived from the adrenal gland. Thus, its elevation highly supports the presence of PHEO, especially when plasma or urine metanephrine is 4x above the upper reference limit. Another new promising option is the use of proton single-voxel magnetic resonance spectroscopy (1H-MRS) that can detect the presence of catecholamines in PHEO and, therefore, may correctly point to the presence of this tumor [59–61].
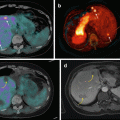
PET imaging using 18F-FDOPA, 18F-FDA PET/CT, or 68Ga-DOTA-SSA PET/CT is highly sensitive with 18F-FDA having an excellent specificity, as described above (Fig. 14.1). The low uptake of 18F-FDOPA by normal adrenals is a potential advantage over 68Ga-DOTA-SSA for localizing a small PHEO (Figs. 14.2, 14.3, and 14.4).
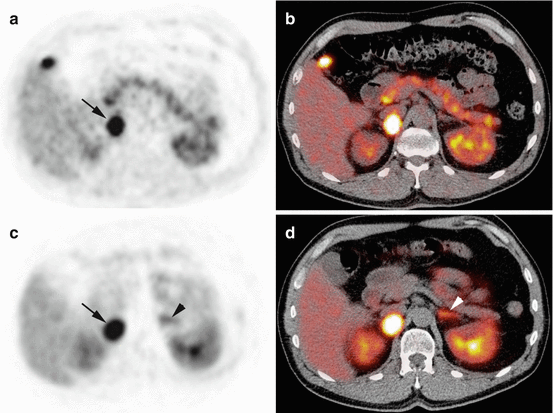

Fig. 14.1




Typical imaging features of PHEO on 18F-FDOPA PET/CT and 68Ga-DOTATATE. Axial 18F-FDOPA PET (a) and PET/CT (b). Axial 68Ga-DOTATATE PET (c) and PET/CT (d). The PHEO was positive on both imaging studies (arrows). Note high 68Ga-DOTATATE uptake by the left normal adrenal gland (arrowheads)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree