Fig. 7.1
Barium esophagogram of a patient with esophageal cancer shows abrupt narrowing of esophagus and focal areas of ulceration (arrow) with stricture
Staging
Clinical staging tools include esophagoscopy with biopsy, EUS, EUS-FNA, CT, and FDG positron emission tomography/computed tomography (PET/CT). Bronchoscopy, cervical lymph node biopsy, endoscopic bronchial ultrasound (EBUS), and EBUS-FNA, ultrasound, or CT-directed biopsies can be used in specific cases [1–4, 6].
CT [8] and EUS have been the mainstay imaging modalities for initial staging; however these modalities may over- or understage as many as 30–40 % of cases [9]. PET/CT demonstrates superiority to other modalities especially for its capabilities in the detection of distant metastasis [9]. Wallace et al. examined multiple imaging modalities for staging and concluded that the preferred staging procedure was PET/CT followed by EUS in cases where no evidence of metastasis was observed by PET/CT [9, 10].
Staging of esophageal cancer has been significantly updated in the seventh edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) cancer staging manuals [4, 11]. Depth of invasion defines the T staging of primary cancer. Tis tumors are intraepithelial without invasion of the basal membrane, currently termed as high-grade dysplasia. T1 cancers extend beyond the basal membrane and invade the lamina propria, muscularis mucosa, or submucosa. T1 cancers can be classified as mucosal (T1a) and submucosal (T1b). T2 cancers breach into but not beyond the muscularis propria. T3 cancers invade beyond the esophageal wall without invading adjacent structures. T4 cancers invade structures adjacent to the esophagus. T4a cancers are still resectable, invading adjacent structures like the pleura, pericardium, and diaphragm. T4b tumors are unresectable due to invasion of other adjacent structures like the aorta, vertebral bodies, or trachea [2–4, 11].
A regional lymph node is defined as any paraesophageal lymph node extending from cervical nodes to celiac nodes. N classification includes N0 (no cancer-positive nodes), N1 (1 or 2 nodes), N2 (3–6 nodes), and N3 (7 nodes or more) [2–4].
Distant metastasis is classified as either M0, no distant metastasis, or M1, distant metastasis. Histopathologic cell type is either squamous cell carcinoma or adenocarcinoma as AJCC/UICC staging is based on cancers arising from the esophageal epithelium. Histologic grade is categorized as G1 well differentiated, G2 moderately differentiated, G3 poorly differentiated, and G4 undifferentiated [3, 4, 11].
Cancer location is expressed as the distance of the proximal end of the cancer from the incisors. This can be correlated with anatomic imaging. If the tumor is above the sternal notch, the esophageal cancer is located in the cervical esophagus. An upper thoracic location on CT corresponds to the region between the sternal notch and lower border of the azygos vein. Middle thoracic tumors are located between the azygos vein and inferior pulmonary vein. The lower thoracic region is below the inferior pulmonary vein (Fig. 7.2) [4, 5, 11].
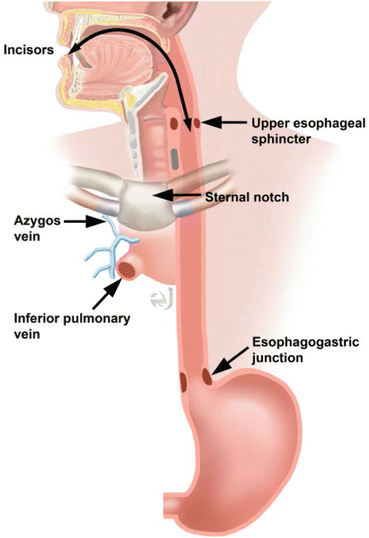

Fig. 7.2
Anatomic localization of esophageal cancer
T Staging
T1 and T2 tumors are generally treated with surgery, whereas patients with T3 and T4 tumors are frequently offered preoperative chemotherapy and/or radiation therapy. Hence, the detection of depth of invasion for proper T staging becomes crucial [1–4, 6].
EUS
EUS is the most accurate imaging tool that provides information about involvement of the esophageal wall that is necessary to define T stage. EUS may detect the involvement of adjacent structures so that it may upstage a cancer to T4 in the presence of extension [5]. The performance of EUS has been shown to improve as the T stage increases [2, 6].
EUS is not accurate in differentiating T from T1. However, US performed with high-frequency probes showed very good results in distinguishing mucosal versus submucosal invasion [1]. In comparison to CT, EUS is more accurate in differentiating between T1, T2, and T3 tumors [2]. However, there are shortcomings of EUS. Like any other sonographic examination, it is operator dependent, and in cases where the esophageal lumen is narrowed, it may be impossible to pass the endoscope through the stricture [2, 3]. In these cases, prior to EUS, mechanical dilatation can be performed; however there is increased risk of esophageal perforation [1, 3].
CT and MRI
Assessment of the esophagus by CT can be challenging especially for T1 and T2 esophageal cancers as detection of a small tumor in a poorly distended tubular structure is quite difficult. Usually, the esophageal wall measures less than 3 mm on CT of a distended esophagus [2, 3]. A wall thickness more than 5 mm is considered abnormal [4].
Asymmetrical thickening of the esophageal wall is a primary but a nonspecific sign [3] for esophageal cancer. CT assessment is less accurate for the detection and staging of esophageal cancer compared to EUS [1–3] (Fig. 7.3).
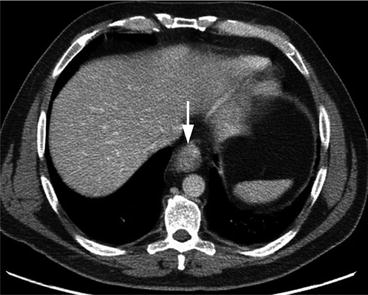

Fig. 7.3
Axial contrast-enhanced CT of the upper abdomen shows marked circumferential thickening of the distal esophagus (arrow), consistent with known esophageal cancer. Unfortunately CT was not able to properly determine T staging as the assessment of esophageal wall layers was limited with this imaging technique
In circumstances when esophagoscopy is not possible, mostly due to the presence of a marked stricture, CT may provide information about the location of the tumor.
The most useful aspect of CT in T staging is to evaluate the presence of invasion of adjacent soft tissues. Direct invasion or obliteration of the fat plane between the tumor and the anatomic structure may indicate local invasion [1–3, 6]. However caution is advised in cachectic patients and in patients with prior history of radiation therapy or surgery as fat planes on CT may not be clearly depicted [1, 2, 6].
In addition, local invasion is suggested by a contact angle of more than 90° between the cancer and the aorta or thickening and displacement or indentation of the posterior membrane of the trachea or left mainstem bronchus, yet neither of these findings is definitive [1, 4, 6]. Finally, tumor extension in the air lumen or a fistula between the esophagus and airway may be visualized; still bronchoscopic confirmation is necessary. Pleural effusion and pleural wall thickening are suspicious findings on CT for tumoral invasion. Direct extension of tumor to the heart or loss of pericardial fat plane can also be detected by CT.
With recent advances in CT technology, it is possible to provide higher quality images with isotropic voxels as well as CT esophagography or virtual endoscopy [3].
Multi-planar reformatted images (MPRs) are useful to estimate tumor length and assessment of the exact location of esophageal cancer is more accurate compared to the axial images only [1, 3]. MPRs are also useful in evaluating esophageal cancers at the esophagogastric junction (EGJ) [1]. Pneumo-CT is a technique developed to image stenotic lesions, optimizing tumor visualization at the esophageal wall [12]. Administrations of effervescent granules, air insufflations, or large amounts of water are other methods to better visualize the esophageal wall by CT [3].
Magnetic resonance imaging (MRI) has limited role in imaging of esophageal cancer due to technical shortcomings. Early studies with MRI demonstrated poor quality especially due to motion artifacts and cardiac/respiratory-related artifacts. Recent developments in cardiac respiratory gating, availability of high field magnets (1.5 and 3 T) for imaging, and the development of new and faster imaging sequences have resulted in better quality images. The addition of sequences such as diffusion-weighted imaging and dynamic contrast enhancement has improved esophageal cancer imaging. Preliminary studies with high-resolution MR imaging report high accuracies for T staging, close to that of EUS [13–15].
FDG PET
The first report in the literature of the use of FDG PET in a patient with esophageal cancer was described in 1995, by Yasuda [9, 16]. Given that FDG PET provides mostly metabolic information about the tumor, determination of T stage is not one of its strengths. Though 92–100 % of esophageal cancers are FDG avid, lack of visualization of esophageal wall layers, even with combined FDG PET/CT, limits accurate assessment of T stage. Some authors, such as Kato, report that T1 tumors lack FDG uptake, likely due to their size below the resolution for PET (0.7–1 cm) [17]. In the study by Kato, it was found that T2, T3 and T4 tumors have similar levels of FDG uptake [18]. Advanced T staging could be seen with combined PET/CT, when the metabolic activity extends to adjacent soft tissues in the mediastinum, and the fat planes are lost suggesting invasion [1].
Increasing data exist to support the use of quantitative measures or metabolic parameters of the primary tumor as prognostic predictors. These include standardized uptake value (SUV), metabolic tumor volume (MTV), and total tumor glycolysis (TLG), among others [18–20]. A meta-analysis of 10 studies and 542 patients by Pan reports that high SUVs are associated with a significantly poorer overall survival and disease free survival. Foley studied these independent predictors of survival, and the most significant was TLG (defined by the product of metabolic volume of primary tumor times SUV mean). In Foley’s study, another significant predictive factor was the “metastatic length of disease” defined as the total length of disease including non-regional lymph node metastases and distant metastases measured in mm. The total count of involved local lymph node metastases on PET/CT was also a significant predictor of survival [19].
Finally, FDG uptake secondary to inflammation from esophagitis may confound accurate T staging, although the pattern of FDG uptake is usually linear and diffuse compared to focal for malignancies [21].
N Stage
Lymphatic involvement can occur at very early stages of esophageal cancer due to the unique bidirectional lymphatic drainage system of the esophagus. The intramural (mucosal) drainage system is located in the lamina propria. Unlike other parts of the gastrointestinal system, this location can result in early dissemination of tumor cells. The second, longitudinal system, is localized in the submucosa, within the muscular layer [2, 3].
EUS
EUS and EUS-FNA are primary tools to identify regional nodal involvement. EUS has an accuracy of 72–80 % [2]. CT has an accuracy ranging between 46 and 58 % [1]. Although EUS is superior to CT in detecting lymph node metastasis, the sensitivity and specificity vary depending on location; for example, detection of celiac axis lymph nodes is better than that of mediastinal lymph nodes with EUS [1].
Combined use of EUS with FNA improves accuracy [1–3]. However, EUS-FNA can only be performed in lymph nodes that are approachable [3]. Metastatic lymph nodes can appear as well-defined (clear border), round, homogeneous, and low-echoic lesions measuring more than 10 mm in diameter [3]. According to Rice et al. [6], the accuracy of detecting nodal metastasis in lymph nodes with all five of these features is 100 % [6]. However, very few metastatic lymph nodes present with all of these findings, especially in a periesophageal location.
CT and MRI
CT provides information about non-regional lymph nodes, mainly supraclavicular, abdominal, and retrocrural lymph nodes. A short axis of more than 1 cm of a lymph node on CT is the most widely used criterion for suspicious lymph node involvement (Fig. 7.4). The cutoffs for retrocrural and supraclavicular nodes are 0.6 cm and 0.5 cm, respectively. However normal-sized lymph nodes may contain tumor deposits, resulting in false-negative examination. Also an enlarged lymph node may not be malignant but could be inflammatory, resulting in false-positive results with CT [2, 3]. Therefore sensitivity and specificity of detection of nodal metastasis with CT are low, with reported accuracy of 46–58 % [2, 3].
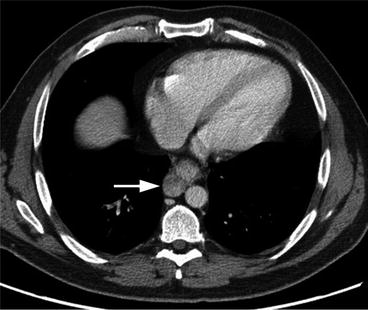

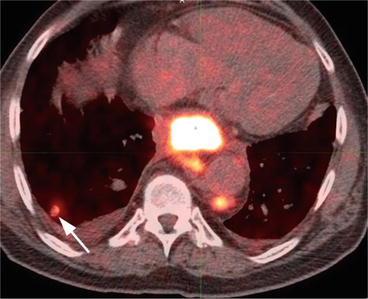
Fig. 7.4
Axial contrast-enhanced CT of the lower thoracic/upper abdomen region shows periesophageal lymphadenopathy, most consistent with malignant lymphadenopathy (arrow) as well as abnormal thickening of adjacent esophageal wall consistent with known esophageal cancer
FDG PET
FDG PET/CT combines the anatomic delineation of CT with PET, which can also identify tumoral deposits by the presence of FDG activity. FDG PET is limited in the detection of locoregional lymph nodes in close proximity to the primary tumor in which intense FDG activity may obscure FDG uptake in small adjacent lymph nodes [1, 4, 19]. The reported sensitivity and specificity for detection of locoregional lymph nodes by PET/CT are 59 and 81 %, respectively, from a meta-analysis of 12 publications [4]. The sensitivity of EUS compared with PET/CT is superior for the detection of lymph nodes, although specificity is lower [8, 24]. The presence of locoregional lymph nodes does not preclude surgery; yet, if lymph nodes are seen beyond these boundaries, such as in the retroperitoneum or upper/mid neck, the patient would be considered to have distant metastatic disease where surgery is contraindicated [25].
Compared to the detection of lymph node metastasis from lung cancer and other cancers, FDG PET/CT is less accurate in esophageal cancer [4]. The addition of FDG PET to EUS-FNA does not change N classification significantly [4]. The sensitivity of PET/CT for the detection of distant nodal metastasis is 90 % [2]. The combined use of PET and CT improves the detection rate of nodal disease (Fig. 7.5). Still, false-positive findings due to chronic inflammation may be a limitation [1].
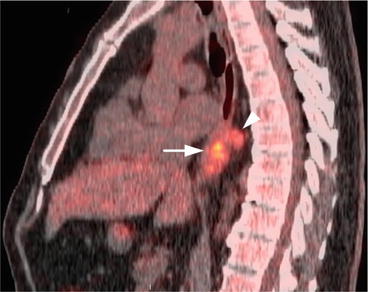

Fig. 7.5
Sagittal fused PET/CT image of the thoracic level shows the hypermetabolic esophageal cancer (arrow) and more cranially located hypermetabolic periesophageal lymph node (arrowhead)
Metabolic parameters have also been used in the evaluation of N staging. A study by Moon evaluated patients with clinically N0 disease and reported that combined use of T classification and SUVmax were strong predictors of occult metastatic disease [26]. Other metabolic parameters such as TLG and MTV have been also studied by different groups. Hsu found a significant correlation between extratumoral maximum SUV and N classification [27].
M Stage
In patients with recent diagnosis of esophageal cancer, 20–30 % will have distant metastasis at the time of diagnosis [1, 2]. Metastases are mostly found in the liver, lung, and bones [1–3, 6]. Except for the brain, contrast-enhanced CT of the chest, abdomen, and pelvis will cover most of the areas that may have metastatic deposits. The most updated National Comprehensive Cancer Network (NCCN) guidelines propose the use of PET/CT in initial staging when upper gastrointestinal endoscopy, biopsy, and CT scan with and without contrast of the chest and abdomen fail to reveal M1 disease [28].
EUS
CT and MRI
Although CT is only 63–74 % sensitive, it remains the mainstay for imaging of distant metastasis [3]. Hepatic metastases are visualized as low-density ill-defined lesions. Contrast-enhanced CT imaging during portal venous phase is mostly used for hepatic metastasis [1]. Lesions less than 1 cm are difficult to detect with CT, which may result in false-negative results [4]. Adrenal metastases usually appear as focal adrenal enlargement or an adrenal nodule. Optimized CT, MR imaging, percutaneous FNA, or laparoscopy may be required to confirm the etiology of these lesions [6, 29].
Solitary pulmonary metastases are rare at initial presentation. Solitary pulmonary nodules are more likely to be either benign or synchronous lung malignancies [5]. Therefore tissue confirmation of solitary pulmonary nodules detected during staging should be considered [3]. Multiple pulmonary metastatic nodules are uncommon at initial presentation, though they are seen more at late stages. CT is very sensitive at detecting pulmonary metastasis. Most pulmonary metastases are round, well defined, and noncalcified [1].
FDG PET
The most common sites for distant metastasis are the liver, lung, bones, and adrenal glands. Less commonly seen are metastases to the brain, subcutaneous tissues, thyroid gland, skeletal muscles, and pancreas [31]. The most important role of FDG PET in esophageal cancer is the detection of distant metastases (Figs. 7.6 and 7.7). As M stage is a major determinant of treatment planning, PET/CT performed at initial workup is becoming standard of care [1].