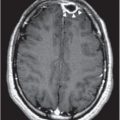
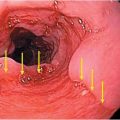
Figure 192.1 Histopathology of human rabies encephalitis. (A) Eosinophilic Negri bodies in a Purkinje cell. (B) Anterior horn cell immunostaining for rabies virus. (C) Purkinje cells immunostaining for rabies virus antigen.
Clinical symptoms
The clinical course in humans is acute, usually progressing from initial symptoms to death within 2 to 3 weeks, even with intensive supportive care. The incubation period of rabies can vary from a few days to several years with an average of 1 to 2 months. The length of the incubation period varies with the infecting strain and is thought to be inversely related to the size of the inoculum and the proximity of the bite to the CNS. Approximately half of the patients develop pain or paresthesias at the wound site. A diagnosis of rabies should not always depend on a history of an animal bite. Exposure may not be obvious in bat-acquired rabies, which often gets misdiagnosed. The prodrome includes low-grade fevers, loss of appetite, and anxiety. Most patients have “furious rabies” characterized by marked hyperactivity, disorientation, hallucination, or bizarre behavior, which lead to an initial psychiatric evaluation. This hyperactivity later becomes intermittent and may be spontaneous or precipitated by tactile, auditory, or visual stimuli. Hydrophobia (spasm of the pharynx and larynx provoked by drinking or the sight of water) and aerophobia (similar effect produced by blowing air on the face of the patient) are considered hallmarks of the disease. The inability to swallow from paralysis of bulbar muscles may also result in hypersalivation. Seizures, which are more common in children, may also occur during this stage, as can dysfunction of the autonomic nervous system. Rarely, there may be respiratory distress with medullary involvement. A few patients die during this stage, but most go on to develop progressive paralysis and eventually coma. Abnormal cranial nerve, motor, and sensory examinations; tremor; myoclonus; and local sensory symptoms at the exposure site are more common in bat-acquired rabies. In some patients, the paralytic state dominates the entire clinical picture; hence, it is termed paralytic rabies. Paralysis or paresis involves the proximal muscles and can be accompanied by constipation, urinary retention, and respiratory failure. Alternatively, inflammation with demyelination and axonal dysfunction of the peripheral nerve causes an ascending lower motor neuron weakness without anterior horn cell involvement. Physical exam shows a motor weakness involving the respiratory muscles and loss of deep tendon reflexes with maintained consciousness which may mimic an acute axonal Guillain-Barré syndrome. This lack of involvement of spinal cord motor neurons and brainstem is called an “escape phenomenon.” In some patients, however, the clinical manifestations can be nonspecific; hence, in all patients with unknown cause of progressive encephalitis, the possibility of rabies should be considered. In patients receiving intensive supportive care, the average duration of illness between onset of paralysis and death is 7 days. Once neurologic symptoms have developed, survival is rare.
Diagnosis
Prior to available accurate laboratory tests, a potentially infected animal was placed in isolation for observation. If it died a characteristic rabies death, the diagnosis was established. With the advent of the microscope in 1903, intracytoplasmic inclusion bodies were discovered in infected brain tissue by Aldelchi Negri, who at the time was an assistant of Camillo Golgi. These structures were subsequently called Negri bodies. In the 1980s the direct fluorescent antibody (DFA) test was formed, which remains to this day the diagnostic gold standard (Figure 192.1). For postmortem diagnosis in animals, fresh unfixed brain tissue is necessary for DFA testing as fixed tissue with agents such as formalin may yield inaccurate results. Brain tissue is the only sample tissue type for diagnosis as saliva and salivary gland excretion of the virus can be intermittent. The predictive value of a negative brain DFA test is 100%.
Routine laboratory tests and diagnostic studies are of little value in the diagnosis of rabies. Examination of the cerebrospinal fluid (CSF) may show leukocytosis, but protein and glucose assays are often normal. Antibodies to the virus in an unvaccinated patient with encephalitis confirms the diagnosis. In addition, saliva samples can be cultured and then tested for viral nucleic acid. A tissue diagnosis can be made in premortem humans using the DFA test on a skin sample from the back of the neck. Polymerase chain reaction (PCR) can also be used in diagnosis, but DFA is still considered the gold standard as PCR is hampered by obtaining universal Lyssavirus primers. Due to the ability to detect low copy numbers of viral nucleic acid, rapid turnaround time, and falling costs, it is likely that PCR-based techniques may become a viable diagnostic test in the near future.
In 2006, the CDC reported a case of encephalitis in a 15-year-old girl from Wisconsin who was bitten by a bat. Antibody titers to rabies virus were found in CSF and serum. She survived the infection. Another young boy developed encephalitis of undetermined etiology that progressed rapidly. He developed rabies-specific immunoglobulin G antibodies in increasing titers. Rabies virus could not be detected in the CSF by PCR; however, antibodies to the rabies virus were present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree