44 Pseudoprogression After Glioma Therapy
Since the introduction of concomitant temozolomide chemo-therapy with radiation for patients with newly diagnosed glioblastoma, there has been increased awareness of post-treatment changes detected on imaging studies, especially with magnetic resonance imaging (MRI). The appearance of increased contrast enhancement accompanied by increased cerebral edema, with or without accompanying clinical symptoms, is seen in 20 to 50% of patients who complete chemo-radiation.1,2 These changes are indistinguishable from those of true disease progression, yet some, and perhaps most, of these changes resolve over time and therefore represent a transient effect of therapy now coined pseudoprogression.3 Most prospective case series have found that patients who experience imaging pseudoprogression have longer overall survival times, so rather than being an ominous sign of treatment resistance, the changes seen following chemoradiation are, paradoxically, sometimes a sign of improved treatment sensitivity.2,4
Pearl
• Pseudoprogression may be a form of treatment response. Worsening appearances on conventional imaging (computed tomography [CT], MRI) within 12 weeks of radiation therapy must be interpreted with caution as not all patients with “worse” scans will turn out to have “worse” disease.
 Definition of Pseudoprogression
Definition of Pseudoprogression
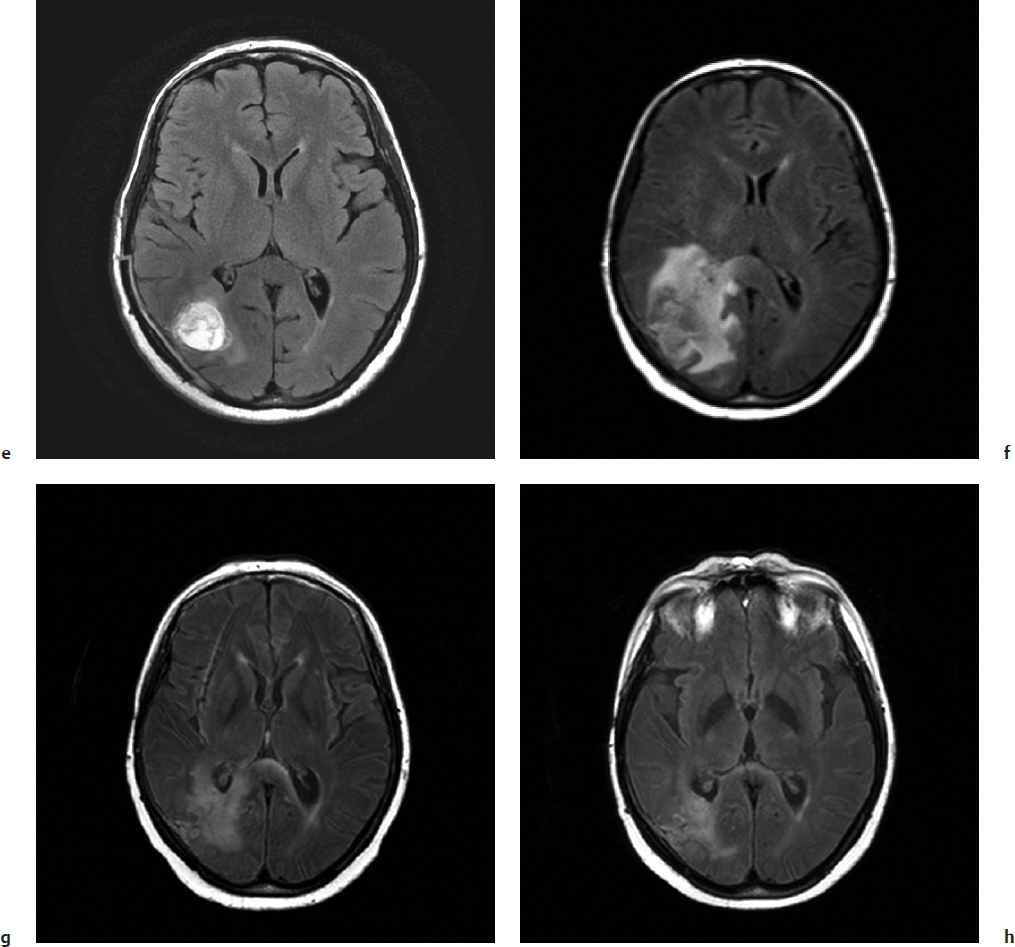
Pseudoprogression is suspected on clinical grounds when post-treatment MRI scans demonstrate features of disease progression such as increased contrast enhancement and edema that later resolve without a change in treatment (clinically suspected pseudoprogression, Fig. 44.1) or, in cases where surgical re-resection is performed, the tissue is found to be inflammatory without clear evidence of viable tumor cells (surgically confirmed pseudoprogression, Fig. 44.2). The vast majority of cases reported in the literature are clinically suspected rather than surgically confirmed; thus, there is much that remains speculative and controversial about the incidence and pathophysiology of this important posttreatment finding.
Until recently, the accepted best practice for determination of tumor response on imaging has been the comparison of measurements using the bidimensional cross-sectional area of the enhancing component of a tumor on consecutive scans.5 These criteria rely on the presence of enhancing disease and also consider the patient’s clinical condition and corticosteroid dose. These criteria have served us well in practice and as a standardized measurement across clinical trials; however, a variety of advances in neuro-oncology have called for a new definition of tumor response.6 For example, not all tumors have contrast enhancement, such as most low-grade gliomas, and some therapies, notably the anti–vascular endothelial growth factor (VEGF) agent bevacizumab, can greatly reduce contrast enhancement, and disease progression can be due to non–contrast-enhancing tumor growth such as increases in T2 or fluid-attenuated inversion recovery (FLAIR) signal on consecutive MRI scans. For these reasons a working group developed updated criteria for response assessment in high-grade glioma termed the Response Assessment in Neuro-Oncology (RANO) criteria,7 which specifically address the issue of pseudoprogression by calling into question the reliability of conventional MRI within 12 weeks of the completion of chemoradiation in newly diagnosed patients. Within these first 12 weeks a diagnosis of disease progression can be made only if an enlarging lesion is outside of the high-dose volume of radiation therapy (RT) or if the enlarging in-field lesion is resected and confirmed to contain viable tumor. Thus, pragmatically, pseudoprogression is an entity commonly considered within the first 3 months after chemoradiation. The cutoff of 3 months is expected to be widely incorporated into clinical trials; however, clinicians are advised to be aware that cases of both clinically suspected and surgically confirmed pseudoprogression have been observed well beyond 3 months.3
 Incidence
Incidence
Pseudoprogression has been widely reported from many institutions with reasonably concordant estimates of frequency. Typically, MRI scans are obtained 4 to 6 weeks following the completion of RT or chemoradiation and are compared to the postoperative MRI or MRI scan acquired at the time of RT planning. It is important for treatment centers to adopt a standardized approach to this sequence of imaging to ensure valid comparisons. For example, the imaging modality of choice is MRI with gadolinium contrast using standard anatomic imaging sequences, ideally with diffusion-weighted MRI to detect postoperative ischemia or infarction that may be confused with enhancing disease. The post-chemoradiation scan should include the same imaging parameters. Early disease progression is suspected when an increase in contrast-enhancing disease is noted. To date, there are no validated criteria to guide how best to conduct tumor measurements at the end of chemoradiation. Most series, and now most clinical trials, suggest using the RANO criteria and consider a 25% increase, or greater, in maximal biaxial diameter to be significant. No clear definition regarding changes in FLAIR imaging has emerged.
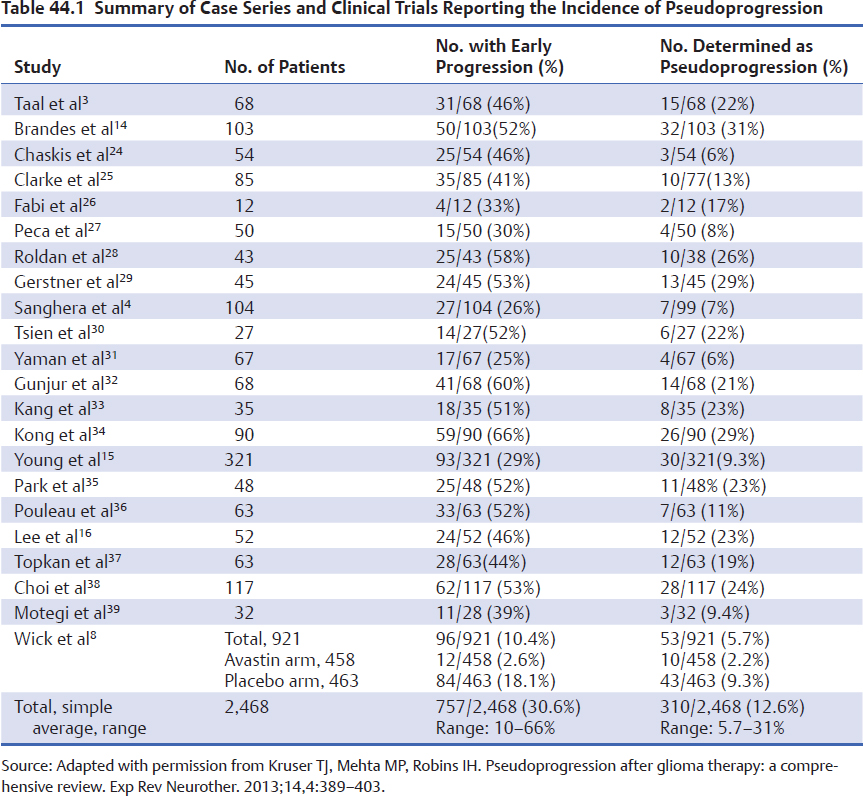
Table 44.1 lists the reported case series of patients who were found to have early imaging progression following chemo-radiation for glioblastoma. Very few of these series have many patients with surgically confirmed pseudoprogression; most of them base the diagnosis of pseudoprogression on subsequent serial imaging that demonstrates stability or improvement of the lesion size over time. Thus, this collection of cases is not standardized with respect to timing of scans or definition of disease; however, recognizing these limitations, we attempted to pool the results across studies. Of the 2,468 patients reported, 30.6% (range, 10-66%) had early progression on the first posttreatment MRI, and of the same 2,468 patients, 12.6% (range, 5.7–31%) were determined to have pseudoprogression based on the lack of imaging progression on subsequent scans without a change in therapy. A very instructive preliminary report from the AVAglio phase III trial of chemo-radiation with RT plus temozolomide with or without bevacizumab versus placebo should be noted.8 Bevacizumab is a potent monoclonal antibody against VEGF ligand and has profound effects on tumor vasculature. Some have termed this effect “vascular normalization,” the net effect of which is felt to be normalization of the “leaky” intratumoral vessels such that less contrast extravasation occurs. Some authors have termed this appearance “pseudoresponse” because MRI subsequent to bevacizumab therapy often shows significant reduction in contrast enhancement and tumor size.9 The AVAglio trial had standardized timing and MRI sequence acquisition, so these data are among the most robust imaging data in the field of neuro-oncology.
Importantly, the diagnoses of pseudoprogression or true disease progression were made only in hindsight once the natural history of the patient’s tumor was seen on serial imaging. This study provides a useful working definition for pseudoprogression: “a > 25% increase in index lesions and/or unequivocal progression of existing non-index lesions relative to baseline and, in the absence of clinical deterioration, was assessed as pseudoprogression, and then confirmed at the end of 2 months of continuing therapy.” In this trial only 12/458 (2.6%) of patients in the bevacizumab-containing treatment arm were felt to have early progression, whereas 84/463 (18.1%) had early progression in the placebo-arm.9 Thus, bevacizumab clearly influences the degree of contrast enhancement seen on imaging and, notably, the 18.1% incidence of early disease progression in the placebo-treated patients was lower than the simple pooled average in other series where imaging criteria were less stringent. The finding of pseudoprogression was similarly lower in the AVAglio trial than suspected from prior mixed series, and, interestingly, the patients in the bevacizumab-containing arm had a very low rate of early progression and pseudoprogression overall (less than 3%).
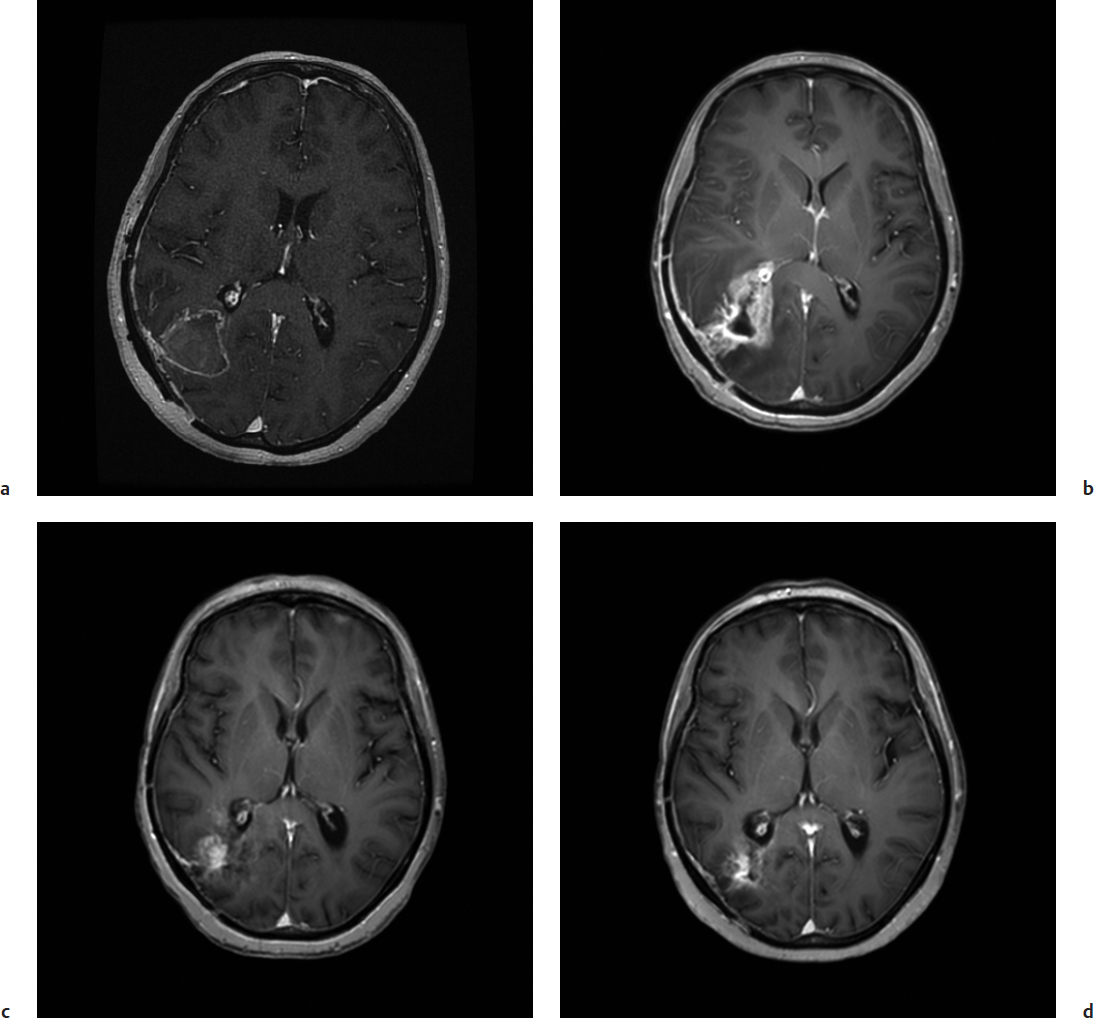
Fig. 44.2a–d Surgically confirmed pseudoprogression following chemoradiation for newly diagnosed glioblastoma in a 54-year-old woman (not the same patient as in Fig. 44.1) with partially excised right parieto-occipital glioblastoma pre- and postradiotherapy. (a) Axial gadolinium-enhanced MRI from the time of radiation planning. (b) Interval development of increased contrast enhancement 6 weeks following completion of chemoradiation. (c,d) Corresponding axial FLAIR images show an increase in FLAIR signal. Taken together these imaging findings are concerning for disease progression. Findings at surgical re-resection demonstrated reactive astrocytosis and inflammation with no viable tumor seen.
Pitfall
• Neuroimaging (CT or MRI) within 12 weeks of the end of radiotherapy is often unreliable. Unless changes in contrast enhancement and edema suggestive of disease progression are outside of the known radiation volume or a new distinct lesion is seen, the diagnosis of disease progression cannot be made with certainty.
 Clinical Significance
Clinical Significance
Pseudoprogression can be seen after many types of therapeutic intervention and in brain tumor types other than glioblastoma. The landmark paper in 2005 changed the standard of care for newly diagnosed glioblastoma.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree