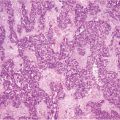
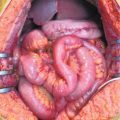
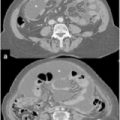
Fig. 16.1
Perforated appendiceal tumor [low-grade appendiceal mucinous neoplasms (LAMN II)]
16.3 Clinical Features
PMP usually manifests with the characteristic jelly belly (Fig. 16.2) appearance, represented by the advanced stage of disease, when most of the abdomen is filled with mucinous ascites and tumor. In fact, cancer cells deposit throughout the entire peritoneal cavity, in contrast to nonmucinous colorectal cancer, where the first metastases are often found near the primary tumor [28]. This kind of presentation can be explained by the so-called redistribution phenomenon consisting of a dissemination pattern associated with intraperitoneal fluid current and gravity. The mobility of the small bowel probably explains why mucus and tumor cells adhere significantly less frequently at these sites, in contrast to more fixed parts, such as antrum, ileocecal region, and rectosigmoid, which are usually massively surrounded by mucus. Usually, patients at this stage present abdominal distension and pain related to obstruction due to the excessive amount of mucinous material. PMP can also manifest with localizing symptoms mimicking acute appendicitis. In female patients, the initial symptoms may be pelvic pressure and palpable ovarian masses. In some cases, mucinous aggregates are discovered incidentally in surgical specimens, such as hernia sacs, thus necessitating the search for the primary neoplasms [29]. Hematogenic or lymphatic dissemination of PMP tumor cells is rare [5, 30–36]
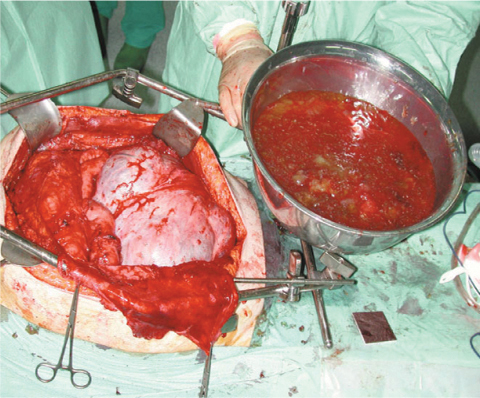

Fig. 16.2
Disseminated peritoneal adenomucinosis [DPAM (jelly belly)]
16.4 Diagnosis
Most patients will have increases in serum tumor markers carbohydrate antigen (CA) 19.9, and carcinoembryonic antigen (CEA), which are useful for both diagnosis and, above all, managing treatment efficacy and recurrence following therapy [37]. Many studies report that preoperative CEA and CA-19.9 levels are useful in predicting disease extent (peritoneal cancer index [PCI]) and surgical success, as well as progression-free (PFS) and overall survival (OS) in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) [38–40] CA-125 is not widely used as a tumor marker for PMP, as it is known to be elevated in a range of benign and inflammatory diseases, thus influencing its specificity. The diagnostic sensitivity of CA-125 for PMP has been examined and approached 60% [41]; the elevated value of this tumor marker seems to be predictive of completeness of cytoreduction and disease-free survival (DFS) [18, 40, 42]. Imaging by ultrasound (US) can be misleading, because the paucicellular mucinous ascites resemble free intraperitoneal fluid [37]; in this case, computed tomography (CT) may be more helpful in preoperative planning by demonstrating the extent of disease. Magnetic resonance imaging (MRI) is scarcely described [43]. Positron emission tomography (PET) in combination with CT might be useful for predicting peritoneal metastasis of high-grade cases [44].
Explorative laparoscopy is an important tool in the diagnosis process: it provides a wide view of the entire abdominal cavity without a large laparotomy, and it may be helpful for definitive histological diagnosis of PMP in patients in whom a primary disease site is not identified. Moreover, laparoscopy allows detection of peritoneal dissemination and establishing ileus involvement from carcinomatosis, which may contraindicate a further laparotomy with curative intent.
16.5 Treatment
Although tumor masses of PMP are often not locally invasive, the mucin is locally destructive. PMP was once treated by iterative debulking operations, which attained unsatisfactory results. Relapses occurred in most cases, and repeated surgeries were more challenging when the disease progressed and scarring, adhesions and distortion of the anatomy developed [45]. A recent study reported the outcome differences between debulking surgery and CRS, both followed by HIPEC, in patients with PMP: CRS and HIPEC were the most efficient treatment modalities, even though associated with a higher morbidity [46].
A serial debulking protocol in patients with limited low-grade appendiceal PMP resulted in a 10-year overall survival of 21–32% [47–49]. Patients with adenocarcinoma who underwent debulking surgery were reported to have a 5- year survival rate of 6% [50] associated with a 30-day postoperative mortality rate of 2.7% [47].
As a result of pioneering work by Sugarbaker, CRS associated with HIPEC have become the mainstays of treatment for PMP [51]. The aim of surgery is complete cytoreduction, as described by Sugarbaker [52], which involves up to six different peritonectomies in combination with visceral resections to remove all macroscopic tumor or, if this is not possible, to leave tumor deposits < 2.5 mm [53]. HIPEC attempts to eliminate microscopic residual disease; complete cytoreduction is attempted, especially at the first operation, as intraperitoneal chemotherapy is used selectively in patients who are able to undergo complete or near-complete surgical cytoreduction.
Chemotherapeutic agents, dosage, temperature, and duration of intraperitoneal chemotherapy have not been subject to randomized trials but have been chosen according to knowledge regarding agents’ intraperitoneal pharmacokinetics. The commonly used intraoperative agents are mitomycin C (MMC), cisplatin, 5-fluorouracil (5-FU), or a combination of these, usually administered for 30–120 min [54]. For early postoperative intraperitoneal chemotherapy (EPIC), 5-FU, and cyclophosphamide are used for up to 6 days [53].
The cornerstone of successful PMP treatment is represented by correct diagnosis and appropriate treatment. Repeated debulking surgery in misdiagnosed disease, often associated with systemic chemotherapy and especially when performed for low-grade malignancies, leads to progressive decrease in intentionto- treat cytoreductive surgery plus HIPEC. Thus, it is mandatory to refer suspect or certain PMP to a trained center. In fact, despite this treatment strategy, the disease, especially if arising from high-grade primary tumors, may recur: early detection of relapse may provide the opportunity for a further complete CRS plus HIPEC, which may prolong survival [55].
16.5.1 Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
There are two main procedures by which to deliver HIPEC: the open-abdomen technique described by Sugarbaker (coliseum technique) and the closed abdomen technique [56]. No significant differences in terms of results between the two techniques are reported in the literature.
Originally, we perform a semiclosed HIPEC technique (using the computerassisted perfusion system PERFORMER HT, Rand), which avoids exposing surgical personnel to chemotherapeutic drugs and could reduce the dissipation that occurs with the coliseum technique, allowing the surgeon to mix the perfusate solution and visually check the peritoneal cavity [57]. The protocols we use are based on administering cisplatinum (CDDP) 100 mg/m2 plus MMC 16 mg/m2 at a temperature of 41.5°C, or MMC as single drug at 35mg/m2 for 60 min at a temperature of 40.5°C, according to the Netherland protocol [58].
16.6 Results
16.6.1 Literature Analysis
Recent evidence suggests that optimal surgical resection (complete cytoreduction if possible) combined with HIPEC is the most fundamentally based strategy for treating PMP. Although this treatment is still associated with a major risk of morbidity, the increase in survival may be acceptable when proposing an alternative to debulking procedures alone. As presented in a recent meta-analysis on the improved survival rate of patients with PMP receiving cytoreductive surgery and HIPEC, the mean mortality rate was 3.75 (median 2.45). The mean and median morbidity reported were 35.75 and 40.0, respectively. Mean 3-, 5-, and 10-year survival rates were 77.18%, 76.63%, and 57.3%, respectively. Median 3-, 5-, and 10-year survival rates were 77.85%, 79.5%, and 55.9%, respectively [59]. In Tables 16.1 and 16.2, the most recent and significant studies and related results in terms of outcome and postoperative complications are reported.
Table 16.1
Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei
Study [Reference] | Median FU (months) | Size (n) | Median OS (months) | 5-year OS (%) | 10-year OS (%) | Median DFS (months) | 5-year DFS (%) | 10-year DFS (%) |
|---|---|---|---|---|---|---|---|---|
Robella et al. (De Simone) 2013 [60] | 35 | 80 | 144 | 89 | 78.5 | 88 | 82 | 68 |
Arjona-Sanchez et al. 2013 [61] | 36 | 38 | 36 | 58.7 | 36 | |||
Chua et al. 2012 [62] (multicentric) | 36 | 2,298 | 196 | 74 | 63 | 98 | ||
Sorensen et al. 2012 [63] | 93 | NR | 79 | 69 | 58 | 47 | ||
Chua et al. 2011 [64] | 34 | 113 | 104 | 79 | 47 | 48 | 47 | |
Youssef et al. 2011 [65] | 456 | 69 | 57 | |||||
Elias et al. 2010 [66] (multicentric) | 88 | 301 | NR | 72.6 | 54.8 | 78 | 56 | |
Alves et al. 2010 [67] | 49 | |||||||
Baratti et al. 2009 [18] | 45 | 102 | 84.4 | 79 | 48.3 | |||
Vaira et al. 2009 [68] | 53 | 94 | 84 | 80 | 70 | |||
Elias et al. 2008 [69] | 48 | 105 | 80.2 | 97 | 68.5 | |||
Smeenk et al. 2007 [70] | 51.5 | 103 | 59.5 | 25.6 | 37.4 | |||
Bradley et al. 2006 [26] | 101 | 57 | ||||||
Gonzalez-Moreno et al. 2006 [71] | 48 | 501 | 156 | 71.9 | 54.5 | |||
Sugarbaker et al. 2006 [72] | 35 | 356 | ||||||
Smeenk et al. 2006 [73] | 35 | 103 | ||||||
Deraco et al. 2003 [74] (multicentric) | 70 | 91 | 54 |
Table 16.2
Morbidity and mortality of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei
Study [Reference] | Procedure | Morbidity (%) | Mortality (%) |
|---|---|---|---|
Robella et al. (De Simone) 2013 [60] | HIPEC | 52.5 | 0 |
Arjona-Sanchez et al. 2013 [61] | HIPEC | 18.4 | 0 |
Chua et al. 2012 [62] (multicentric ) | HIPEC | 24 | 2 |
Sorensen et al. 2012 [63] | HIPEC-EPIC | 24 | |
Chua et al. 2011 [64] | HIPEC-EPIC | 44 | 1 |
Youssef et al. 2011 [65] | HIPEC-EPIC | 7 | 1,6 |
Elias et al. 2010 [66] (multicentric) | HIPEC-EPIC | 40 | 4.4 |
Alves et al. 2010 [67] | HIPEC-EPIC | 9 | 2 |
Baratti et al. 2009 [18] | HIPEC | 0.98 | |
Vaira et al. 2009 [68] | HIPEC | 45 | 0 |
Elias et al. 2008 [69] | HIPEC | 67.6 | 7.6 |
Smeenk et al. 2007 [70] | HIPEC | 54 | 2.9 |
Bradley et al. 2006 [26] | HIPEC | ||
Gonzalez-Moreno et al. 2006 [71] | HIPEC-EPIC | ||
Sugarbaker et al. 2006 [72] | HIPEC-EPIC | 40 | 2 |
Smeenk et al. 2006 [73] | HIPEC | 54 | 3 |
Deraco et al. 2003 [74] (multicentric) | HIPEC | 14 | 1.4 |
16.7 Our Experience
In our Institution, from 1995 to December 2013, ∼800 operations for peritoneal carcinomatosis were performed, and in 350 cases, surgical cytoreduction associated with HIPEC were carried out. We operated about 200 patients presenting PMP; 107 of those underwent CRS plus HIPEC. Inclusion criteria for the procedure were the following:
Presence of tumor confirmed by histology or cytology;
Patients aged between 18 and 72 years;
Performance status (PS) < 2 according to the Eastern Cooperative Oncology Group (ECOG);
No evidence of extraperitoneal disease;
Absence of concomitant uncompensated cardiopulmonary, hepatic, renal, and metabolic disease;
No prior abdominal radiotherapy for carcinomatosis or over a wide abdominal area.
Preoperative evaluation always included thoracic and abdominal CT scan to stage peritoneal disease and exclude distant metastases; upper digestive endoscopy and colonoscopy generally completed tumor staging. A careful preoperative evaluation of the patient’s general conditions was always performed and included complete blood tests, electrocardiogram, cardiac US, and spirometry.
Follow-up data are available for 95 CRS plus HIPEC performed in 86 patients (11 patients were excluded because follow-up was too short or they were lost). With a median follow-up of 41.6 months (range 3–167 months), we reported a 5- and 10-year OS of 81.6% and 67.9%, respectively. Median OS was not reached at 10 years. Five- and 10-year DFS were 61.5% and 45.8%, respectively, with a median of 72 months. Morbidity was 37.9% (36/95), with a major complications rate of 22.1% (21/95). Reoperations were required in 13.7% of cases (13/95), with no perioperative deaths. Our results in terms of OS, DFS, morbidity, and mortality rates are comparable with those reported in the literature.
Twenty-eight patients recurred (20 PMCA and 8 DPAM). Most patients relapsed within 2 years (median 23.7 months). A reiterative CRS was performed in 50% of cases, whereas eight patients were treated with a second CRS plus HIPEC; one patient underwent a third procedure.
Tumor histology (p=0.02), PCI > 20 (p=0.074), and previous systemic chemotherapy (p=0.038) were identified in the univariate analysis as independent predictors for a poorer long-term OS. Preoperative systemic chemotherapy (p=0.008) and PCI > 20 (p=0.001) have a strong negative impact on DFS. In our study, contrary to what is reported in the literature, complete cytoreduction (CC-0 vs CC-1) had a nonstatistically significant impact on outcome. This result may be due to our selection policy, as we do not perform HIPEC if a CC- 2 or CC-3 surgical cytoreduction is obtained. In our casuistry, only 13 patients were CC-1; as a consequence, we did not report a statistically significant impact on OS and DFS regarding this factor.
16.8 Challenging Problems
16.8.1 Recurrence, Treatment, and Repeated CRS plus HIPEC
Despite a much-improved understanding in terms of biology and immunohistochemistry of the disease, the impact of therapy is still incompletely understood; relapses are not uncommon, even if complete surgical cytoreduction is performed, and multiple operations are sometimes required. In the literature, iterative cytoreductive surgery associated or not with HIPEC, presents encouraging results associated with a complications rate comparable with the first procedure. In our experience, disease recurrence occurs within 2 years of CRS plus HIPEC (23.7 months). Histology PMCA is related to relapse in most cases (71.4%). We reported a morbidity rate of 50% (4/8); in one case, reoperation was required for bleeding, but no perioperative mortality was recorded. We detected a 3- and 5-year OS of 77.5% and 34%, respectively (the abrupt decrease is due to the death of two patients from other causes).
A comparable trend is described in the literature, and a long-term DFS is distinctly uncommon, whereas a long-term OS is frequently reported. Some authors reported that most patients recur within 2 years, but a further treatment (CRS, associated or not with HIPEC; or systemic chemotherapy) is feasible, with acceptable morbidity and mortality rates and encouraging outcome results [59]. A paper by Chua [64] who enrolled 113 patients with low-grade PMP, reported a recurrence rate of 41% associated with poor outcome results in early recurrence (<12 months). It is remarkable that patients have been submitted to CRS without repeated HIPEC.
16.8.2 Radiotherapy Treatment for Local Multiple Recurrence
In our experience, 4/28 patients who relapsed presented a repeated, single-site, local recurrence. They were submitted twice to radical surgical removal of relapse, but the disease recurred a third time, and they were treated with radiotherapy at the relapse site. At a median follow-up of 9 months (range 3–13), all four patients were alive without disease progression. In the literature, an interesting paper reported using whole abdominopelvic radiotherapy in palliative treatment for intestinal bowel obstruction caused by recurrent PMP, with resolution of clinical symptoms for 24 months [75].
16.8.3 Treating Appendiceal Mucoceles
The so-called LAMN, divided into LAMN-I and LAMN-II, are often a challenging problem for general surgeons [23]. It appears reasonable, in our experience- and we found a validation in literature reports—to treat LAMN-I disease by appendectomy, associated with multiple peritoneal biopsies and peritoneal washing for cytological examination, histological examination of appendiceal lymph nodes, and margins of resection. We must emphasize that either laparotomic or laparoscopic appendectomy must be performed safely in order to maintain appendiceal-wall integrity. In case of negative biopsies, resection margins, cytology, and lymph nodes, appendectomy is to be considered curative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree