Gastric cancer is the fourth most common malignancy worldwide and the second most common cause of cancer-related death.1 Histologically, gastric cancer may be classified as either of the diffuse type or of the intestinal type. The intestinal type is linked to environmental risk factors and advanced age. The diffuse type (typically associated with signet ring cell histology and/or the clinical picture of linitis plastica in advanced stages) occurs in younger patients and can have a hereditary component. Partly due to a significant decrease in the incidence of the more common intestinal type, the overall incidence of gastric cancer has decreased. However, the incidence of diffuse gastric cancer has either increased or remained stable. Approximately 10% of gastric cancer cases demonstrate familial clustering, and 1% to 3% meet the criteria for hereditary diffuse gastric cancer (HDGC).2,3
Hereditary diffuse gastric cancer is a genetic cancer susceptibility syndrome diagnosed by one of the following: (1) two or more documented cases of diffuse gastric cancer in first- or second-degree relatives, with at least one diagnosed before the age of 50 and (2) three or more cases of diffuse gastric cancer in first- or second-degree relatives, independent of age of onset. The average age of onset of HDGC is 38 years. It is inherited in an autosomal dominant pattern.4
In 1998 inactivating germline mutations in the E-cadherin gene CDH1 were identified in three Maori families with diffuse gastric cancer.5 The CDH1 mutations in these families had an autosomal dominant pattern with high, but not complete, penetrance. Clinically apparent gastric cancer occurred at a young age with the youngest affected individual dying at the age of 14.5 Germline mutations of CDH1 have been detected in 30% to 50% of all patients with HDGC.3,6 More than 50 mutations have been seen across diverse ethnic backgrounds including all nationalities.3 In addition to gastric cancer, patients with germline CDH1 mutations have an increased risk of lobular breast cancer and this may present clinically prior to the diagnosis of gastric cancer in some individuals.7 CDH1 is the only gene that has been found to be present in HDGC. Approximately 70% to 80% of individuals with a germline CDH1 mutation develop diffuse gastric cancer, but this number may be even higher.8
CDH1 is located on chromosome 16q22.1 and encodes the calcium-dependent cell adhesion glycoprotein E-cadherin. Functionally, E-cadherin impacts maintenance of normal tissue morphology and cellular differentiation. CDH1 acts as a tumor suppressor gene in HDGC, with loss of function leading to loss of cell adhesion and subsequently to proliferation, invasion, and metastasis. The germline CDH1 mutation is a truncating mutation. Missense mutations have been reported but their functional significance is unknown. In vitro assays for cellular invasion and aggregation may predict the functional impact of missense mutations.6 Within the gastric mucosa, a “second hit” leading to complete loss of E-cadherin function (Fig. 102-1) probably occurs as a result of CDH1 promoter methylation, as is seen in sporadic gastric cancer.9
FIGURE 102-1
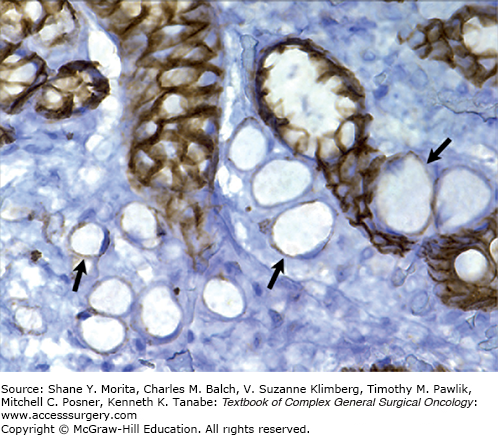
Hereditary diffuse gastric cancer is associated with loss of E-cadherin gene expression. Immunohistochemistry of E-cadherin expression (brown cells) in a patient with HDGC demonstrates that the signet ring cancer cells (arrows) no longer express E-cadherin (lack of brown color). (Reproduced with permission from Norton JA, Ham CM, Van Dam J, et al: CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer, Ann Surg. June 2007;245(6):873-879)
CDH1 mutation screening is recommended in the following situations:
Families with two or more cases of diffuse gastric cancer.
Individuals with diffuse gastric cancer before the age of 40 years without a family history.
Families or individuals with cases of diffuse gastric cancer (one case below the age of 50 years) and lobular breast cancer.
Cases where pathologists detect in situ signet ring cells or pagetoid spread of signet ring cells adjacent to diffuse type gastric cancer.2,10
After obtaining informed consent, a team composed of a geneticist, a gastroenterologist, a surgeon, and an oncologist should discuss the possible outcomes of testing and the management options. Genetic testing should first be performed on a family member with HDGC or on a tissue sample if no affected relative is living. In addition to direct sequencing, multiplex ligation-dependent probe amplification is recommended to test for large genomic rearrangements. If a CDH1 mutation is identified, asymptomatic family members should proceed with genetic testing, preferably by the age of 20.4 If no mutation is identified in a family member with diffuse gastric cancer, the value of testing others is negligible, so further testing is not indicated.
Among individuals found to carry a germline CDH1 mutation, the sensitivity of radiographic and endoscopic screening for gastric cancer has been poor. Histologically, HDGC is in early stages characterized by multiple microscopic infiltrates of malignant signet ring cells that may underlie normal mucosa.11 Because these malignant foci are small in size (typically <4 mm) and diffusely distributed throughout the stomach, they are difficult to identify via random endoscopic biopsy. Endoscopic surveillance for HDGC has been reported previously to be ineffective.3,12 In one large series, preoperative endoscopy failed to detect HDGC in 21 out of 23 (91%) patients.12 However, it has been reported that the addition of chromoendoscopy with Congo red and methylene blue improves the sensitivity of detection of early HDGC.13 Despite its promising results, further use of this technique was curtailed because of concerns over dye toxicity. The lack of a sensitive screening test for HDGC makes early diagnosis challenging. By the time patients are symptomatic and present for treatment, they usually have locally advanced gastric cancer, and their prognosis is poor (Table 102-1).14 Published case reports describe patients who have multifocal diffuse gastric cancer despite normal preoperative endoscopy with random biopsies.15 Although asymptomatic CDH1 carriers who undergo prophylactic gastrectomy are typically cured, the 5-year survival rate for individuals who develop clinically apparent diffuse gastric cancer is only 10%, with the majority dying before age 40.14
Outcome of Patients with Hereditary Diffuse Gastric Cancer Based on the Presence of Symptoms at Diagnosisa,b
| n | Symptoms | Age, Years (Range) | Sensitivity of Endoscopy | 4-Year Disease-Free Survival (%) | 4-Year Disease-Specific Survival |
|---|---|---|---|---|---|
| 13 | No | 48 (18–70) | 15% | 100% | 100% |
| 5 | Yes | 40 (23–52) | 100% | 20% | 40% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






