Figure 81.1 An abnormal infected oligodendrocyte with enlarged nuclei.
Epidemiology
The epidemiology of PML may be divided into several epochs. These include the pre-description era (prior to 1958); the pre-AIDS era (1958–1981); the AIDS era (1981–2005); and the monoclonal antibody era (2005 to present). Prior to 1982, 200 cases of PML had been recorded by the National Center for Health Statistics and an extensive review of the literature published in 1984 was able to find but 230 cases. The overwhelming majority of these cases were the consequence of lymphoid (predominantly B cell) malignancies. Other neoplastic disorders, granulomatous disease, such as tuberculosis and sarcoidosis, and immunosuppressed conditions followed in frequency. In early series, about 5% of patients with PML had no identifiable underlying predisposing disorder, but this is likely to be an overestimate of the true incidence, as illnesses such as idiopathic CD4 lymphopenia had yet to be described. From the beginning of the AIDS pandemic through the introduction of HAART, the numbers of PML deaths has increased dramatically. From 1981 to 1990, 0.73% of AIDS deaths reported to the Centers for Disease Control and Prevention were associated with PML. However, most series suggest that approximately 5% of HIV-infected individuals ultimately develop PML. A striking 20-fold increase in the prevalence of PML was seen between the years 1980 to 1984 and 1990 to 1994 in south Florida with all but 2 of 156 cases of PML in this series occurring in association with HIV. Typically, AIDS patients with PML have significant lymphopenia and low CD4 lymphocyte counts; however, in one series, >10% had CD4 counts in excess of 200 cells/mm3 at the time of presentation. The introduction of HAART may have led to a decline in the frequency with which PML complicates HIV infection, although this remains to be unequivocally established.
Clinical manifestations
The clinical manifestations of PML are varied and depend on the area of the brain involved. In natalizumab-associated PML, the most common abnormalities were behavioral and cognitive abnormalities followed by weakness; whereas, in the HIV-associated PML cases, the most common manifestations have been weakness (generally, hemiparesis), gait disturbance, speech and language disorders, cognitive dysfunction, and visual loss. Ataxia, dysarthria, numbness, headaches, aphasia, seizures, and vertigo are occasionally noted. Rarely, focal cognitive deficits, such as prosopagnosia, apraxia, left-sided neglect, and Gerstmann’s syndrome, are observed; however, global deficits such as memory disturbances and personality changes are more common. There may be some differences in clinical presentation of PML based on the predisposing cause. On rare occasion, magnetic resonance imaging (MRI) abnormalities due to PML may be detected in advance of any clinical features; however, symptoms typically intervene within weeks of this observation.
Radiology
The diagnosis of PML is strongly suggested by the typical appearance on imaging studies. On computed tomography (CT), multiple white matter hypodensities are revealed (Figure 81.2), but MRI is more sensitive. The lesions of PML appear hyperintense on T2 (Figure 81.3) and hypointense on T1. The scalloped appearance of these areas is due to subcortical “U” fiber involvement. Although any area can be affected, there is a predilection for the frontal and parieto-occipital regions, perhaps due to the large volume of white matter in these areas. About one-third of patients have posterior fossa involvement, and 5% have only cerebellar and brainstem lesions. Most patients have bilateral abnormal areas, and the basal ganglia may be affected, chiefly due to involvement of myelinated fibers that course through this region. Enhancement had not been considered typical, but in a pre-HAART AIDS PML population, 10% of patients had contrast enhancement on CT scan and 15% had gadolinium enhancement on MRI. In natalizumab-associated PML, 40% to 50% have gadolinium enhancement on MRI.
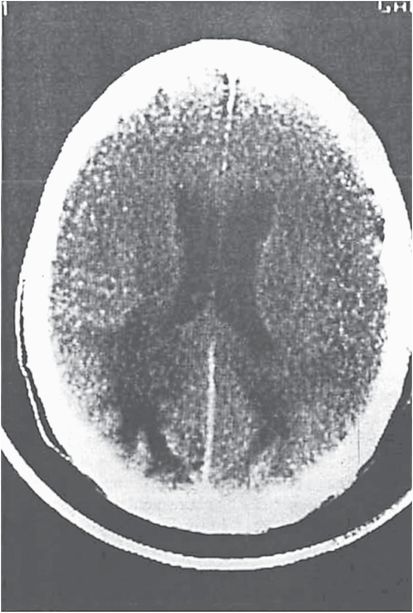
Figure 81.2 Computed tomography scan shows hypodense abnormalities in bilateral occipital lobes.
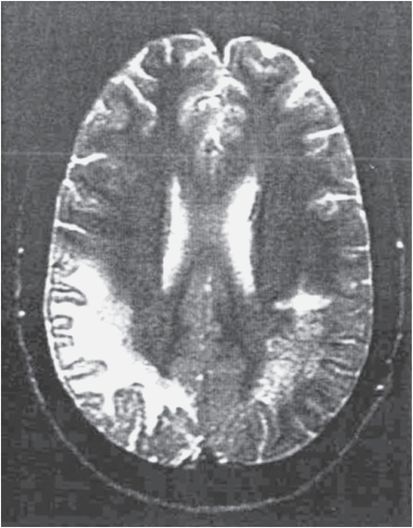
Figure 81.3 This T2-weighted magnetic resonance image shows extensive hyperintense signal abnormalities in the right hemisphere white matter and smaller subcortical lesions on the left.
When PML occurs in association with HIV infection, it must be differentiated from HIV leukoencephalopathy, although this can be difficult on a radiologic basis. The MRI of HIV encephalopathy often shows atrophy and the white matter lesions do not enhance and are typically isointense on T1-weighted imaging. Clinical distinguishing HIV PML characteristics are its rapid course, focal features, and subcortical involvement. In contrast, HIV encephalopathy or dementia has a more protracted course, is of a cortical nature, and only rarely has focal features.
In patients developing PML while under treatment for MS with natalizumab, the white matter lesions of MS must be distinguished from those of PML. This can be difficult but the presence of periventricular lesions, ring, C-shaped or uniform globular enhancement, and Dawson’s finger appearance are among the features that suggest MS, whereas subcortical location, faint, irregular contrast enhancement, a sharp border toward the gray matter and an ill-defined border towards the white matter on T2-weighted images, and T1-hypointensity and diffusion-hyperintense lesions are those suggestive of PML.
Cerebrospinal fluid
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





