© Springer International Publishing Switzerland 2015
Nabil F. Saba and Bassel F. El-Rayes (eds.)Esophageal Cancer10.1007/978-3-319-20068-2_99. Principles and Approaches in Surgical Resection of Esophageal Cancer
(1)
Thoracic Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Despite the scarcity of level I evidence, many strongly held opinions persist regarding best practices for the surgical management of esophageal and gastroesophageal junction tumors. The range of opinions span from a seeming nihilism, wherein the role of esophagectomy is considered to be primarily palliative, to a belief in “radical” resections, wherein an en bloc esophagectomy constitutes the major component of a curative treatment strategy. Fortunately, some data have accrued during the past decade that can help clarify at least some of the principle areas of importance in esophageal resection. This new knowledge includes clarification of the variable risks of developing nodal disease, on the basis of tumor stage, and what role the assessed risk should play in the choice of operation, the importance of operative margins, and the oncologic implications of technical complications. In addition, there is now a better understanding of what is truly necessary in an esophagectomy and what is lore. Ultimately, if these principles are considered when choosing a surgical approach, some of the controversies regarding esophagectomy should be resolved.
The Balance Between Technical Complications and Outcomes
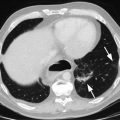
Esophagectomies have historically been associated with a high incidence of morbidity and mortality, much of which can occur as a consequence of intraoperative technical complications, including anastomotic leaks, conduit failures, strictures, thoracic duct leaks, and recurrent nerve injuries. Some of these technical complications can be attributed to poor technique; others, however, are likely related to aspects of the surgical approaches discussed in this chapter. Although advances in pain management, respiratory physiotherapy, endoscopic and radiologic interventions, and intensive care management have all contributed to the overall improvements in morbidity and mortality seen in recent years, technical complications and their consequences persist. In addition to carrying immediate perioperative risks, technical complications can also have an insidious, long-lasting effect on survival, one that is masked by short-term improvements [1]. Consequently, when choosing an operative approach, consideration should be given to the incidence of the technical complications associated with the chosen approach, and the risk of these complications should be balanced against the oncologic and long-term symptomatic benefits of the approach.
In general, the aspects of esophagectomy that have been associated with a higher risk of complications include the use of thoracotomy, a more radical resection, a more extensive lymphadenectomy, and a cervical incision. Thoracotomies have been consistently associated with a higher risk of pneumonia, a finding seen in both retrospective studies [2] and a prospective randomized trial [3]. Likewise, more radical resections require longer operative times, which have been associated with increased incidence of respiratory complications [4]. More extensive lymphadenectomies also require longer operative times and are more likely to result in chyle leak. Last, rates of anastomotic leak and stricture are consistently higher the more proximal the anastomosis is: when the anastomosis is performed in the neck, the incidence of leaks is 8–15 %, compared with 0–7 % when the anastomosis is performed in the chest [5]. Furthermore, the more proximal the anastomosis, and when a cervical incision is used, the greater the likelihood of a recurrent nerve injury.
What is obvious regarding the associations between various aspects of esophagectomy and complications is that the aspects of an operation that are needed to achieve appropriate oncologic goals are often in direct conflict with the need to minimize technical complications. Thus, when selecting an operative approach, there needs to be careful consideration of both the risks of the operation and the oncologic benefits. Clearly, this would imply that, for some patients for whom an aggressive approach is unlikely to result in a significant oncologic benefit, the associated risks of aggressive surgery are not warranted. On the other hand, for patients who can gain a clear benefit from a more radical resection, an aggressive approach is likely worthwhile.
Operative Nomenclature
There are many possible surgical approaches for performing an esophagectomy, with the nomenclature variably describing the number of cavities opened (McKeown, Ivor Lewis, left thoracoabdominal, and transhiatal), the extent of lymphadenectomy (1-field, 2-field, modified 3-field, and 3-field), and the radicality of the resection (en bloc). With the introduction of minimally invasive techniques, a description of the type of access incision is now also used (open, minimally invasive, minimally invasive “assisted,” and robotically assisted). Whereas the components of an en bloc resection are well described—and include resection of the pericardium, contralateral pleura, azygos vein, thoracic duct, and cuff of diaphragm (in junction tumors) [6]—the extent of lymphadenectomy is less well characterized. A “1-field lymphadenectomy,” which refers to an intra-abdominal lymphadenectomy, does not define precisely which nodes need to be removed. Whereas many would agree that the equivalent of a gastric D1 lymphadenectomy is appropriate (N1 nodes − immediate perigastric nodes), others would argue for a more thorough approach, such as a D2 lymphadenectomy (N1 nodes + left gastric, celiac axis, common hepatic, splenic hilum, splenic artery, and hepatic artery nodes) [7]. The second field of a lymphadenectomy commonly refers to an infra-azygos nodal resection, including the subcarinal nodes and all periesophageal nodes from the subcarina down to the level of the hiatus [8]. Last, the terminology used to describe the third field of a lymphadenectomy is inconsistent. A standard third field includes the intrathoracic component along the left and right recurrent laryngeal nerves, as well as a cervical component in which the remaining recurrent nerve nodal chain and the deep cervical nodes are removed [7]. An extended third field also includes the bilateral cervical and supraclavicular nodes [9]. Equally ambiguous in the terminology used to define lymphadenectomy is the designation of a “minimally invasive” approach. For instance, a “minimally invasive Ivor Lewis approach” could imply laparoscopic and thoracoscopic incisions, but for some, the use of either a laparotomy or a thoracotomy would still qualify as a minimally invasive “assisted” approach. Finally, although a surgical approach can limit the extent of the lymphadenectomy or the radicality of the procedure, the name of the approach does not necessarily imply the extent of the lymphadenectomy or the radicality of the operation. For instance, whereas a McKeown esophagectomy allows for a 1-, 2-, or 3-field lymphadenectomy with or without an en bloc component, an open transhiatal esophagectomy does not allow for a formal second- or third-field lymphadenectomy or an en bloc resection. Consequently, the most accurate way to describe an operative approach is by a combination of descriptors; examples would include “a minimally invasive, McKeown esophagectomy with a 2-field lymphadenectomy with thoracoscopy and laparotomy” or “an open Ivor Lewis esophagectomy with en bloc resection and a 2-field lymphadenectomy.”
Principles: Importance of Lymphadenectomy
There is accumulating evidence supporting an association between a more aggressive lymphadenectomy and survival in surgically treated patients with esophageal cancer [10–14]. This association is strongest in patients with the highest risk of nodal disease [12]. That is, in patients who are least likely to have nodal metastases, fewer than 10 nodes should be removed; in patients with a high risk of nodal disease, more than 30 nodes should be removed; and in patients with almost no risk (T1a) of nodal disease, as well as patients with many involved nodes (N3), removal of additional nodes has little bearing on survival. This better understanding of tumors with low and high risks of nodal metastases provides the necessary oncologic rationale for endoscopic resection of superficial tumors (T1a). Conversely, most patients currently undergoing surgery have a moderate to high risk of nodal metastases, and performing an adequate lymphadenectomy in these patients mandates an aggressive approach. Level I support for this approach comes from the only prospective randomized trial that has addressed the effect of surgical approach on survival. This trial compared a “radical” transhiatal approach that is much more thorough than the typical transhiatal esophagectomy with an en bloc Ivor Lewis esophagectomy. The main difference between the two arms of this trial was the number of nodes removed (31 for en bloc, 16 for transhiatal). The results showed a trend toward improved survival for the en bloc Ivor Lewis approach, compared with the radical transhiatal approach [3]. Furthermore, an unplanned subgroup analysis found a statistically significant survival benefit in patients with one to eight nodes involved [15], which is consistent with the concept that patients at risk of nodal disease benefit most from an aggressive lymphadenectomy.
When selecting a surgical approach, the choice should therefore take into account the need for an adequate lymphadenectomy. In some cases—for instance, when an endoscopic resection is not feasible—an approach that achieves a limited lymphadenectomy, such as a transhiatal approach, is adequate. On the other hand, in most patients, an adequate lymphadenectomy will consist of an aggressive intra-abdominal and intrathoracic lymphadenectomy (i.e., 2-field). The intra-abdominal component should include the celiac, splenic, and common hepatic lymph nodes, and the intrathoracic lymphadenectomy should include all the lymph nodes from the subcarinal space down to the hiatus. Because of these requirements, an intrathoracic approach is necessary to adequately remove all potentially involved lymph nodes, and an approach such as a transhiatal esophagectomy should be considered to be inadequate for these patients. It should be noted that an appropriate lymphadenectomy allows for either open or minimally invasive approaches, as long as an appropriate lymph node dissection is achieved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree