Fig. 10.1
Contrasting strengths and weaknesses of both anatomic and molecular imaging. The fusion of both modalities with hybrid PET/CT or PET/MRI enables the evaluation of function and anatomy contemporaneously with precise co-registration between the two modalities
In the conventional cancer imaging paradigm, anatomic imaging is used to define the number and size of lesions. From these, a tumor stage can be derived, typically using the American Joint Committee on Cancer (AJCC) staging system which classifies tumors into different groups depending on the extent of the primary tumor (T stage), nodal spread (N stage), and distant metastatic disease (M stage). To define whether there is nodal disease involvement, arbitrary size criteria are utilized with anatomically enlarged nodes considered likely malignant.
The molecular imaging paradigm for tumor imaging is contrasted by focusing on MNT stage emphasizing that once distant metastases are identified, the locoregional staging is less important (Fig. 10.2). Molecular imaging also performs well in the conventional paradigm of identifying lesions and measuring them, particularly given the high tumor-to-background contrast that is frequently observed. The high tumor-to-background contrast seen with PET/CT also ensures high reporter agreement. There is less reliance on size, since normal size structures that have abnormal uptake can be characterized as malignant. With highly specific radiotracers and modern generation PET equipment, structures smaller than 5 mm can be confidently identified as malignant if they have high uptake. Given the advantages of PET/CT, we advocate that it should be utilized early in the patient workup as the first rather than last imaging modality [1].
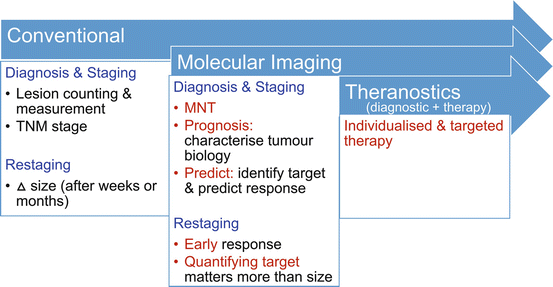

Fig. 10.2
The changing cancer imaging paradigm with the use of molecular imaging. This archetype is particularly well demonstrated for neuroendocrine tumors where somatostatin receptor (SSTR) expression assessed with imaging serves as a predictive biomarker for likely response to somatostatin analogue (SSA) therapy or peptide receptor radionuclide therapy (PRRT). FDG retains a pivotal role for assessing tumor biology with dedifferentiated tumors having high glycolytic activity and poor prognosis
The paradigm shift and advantages of molecular imaging, however, go beyond its ability to identify, count, and measure lesions. Through its ability to characterize tumor biology, it provides powerful prognostic information [2]. This was previously the realm of histopathology, but with the insights of PET/CT imaging, the limitations of relying on characteristics of biopsy from a single site are becoming clear. This is particularly true for metastatic neuroendocrine tumors where their inter-tumor heterogeneity may be observed with varying degrees of tumor differentiation at different sites. In addition to its prognostic power is the predictive power of molecular imaging. This is particularly well demonstrated in theranostic treatments, in which the same molecule is labeled to a therapeutic beta emitter rather than a gamma or positron emitter. The diagnostic scan thereby serves as a predictive biomarker to determine suitability for targeted therapy. This is a well-grounded principle that has been used to successfully treat patients with thyroid cancer for more than 75 years.
10.2 PET Versus SPECT and Selection of Radiotracer
For diagnostic imaging, PET has clear technical superiority compared to SPECT including higher sensitivity for detecting radioactive decay, higher spatial resolution, higher temporal resolution, quantitative capability, and, often, lower radiation dose to the patient owing to the use of short half-life radiotracers [3]. In our center, PET has become the dominant modality for cancer imaging. For imaging neuroendocrine tumors, we employ a variety of radiotracers predominantly using fluorine-18 (18F) and gallium-68 (68Ga) labeled to fluorodeoxyglucose (FDG) and peptides that bind to SSTR, respectively. The latter including DOTATATE, DOTATOC and DOTANOC, which have variable affinity for different subtypes of SSTR, but in clinical practice have similar performance [4]. We have a preference to use DOTATATE as we also treat patients with Lu-177 (177Lu)- and Y-90 (90Y)-radiolabeled DOTATATE, and it therefore serves as a theranostic pair. The short half-life of 68Ga of 68 minutes means that imaging must be perform within a few hours of injection, negating the ability to perform delayed imaging (e.g., at 24 h) that can be readily performed with Indium-111 (111In)- or Technetium-99m (99mTc)-radiolabeled octreotide with SPECT imaging. The early time-point imaging with 68Ga is not suitable for performing prospective dosimetry where imaging at 24-h or later time-points are required. Positron emitters with longer half-life such as Copper-64 (64Cu) are better suited for prospective dosimetry but are not in widespread use. Carbon-11 (11C) labeled to 5-hydroxytryprophan can also be used to image neuroendocrine tumors [5], but their clinical implementation is not widespread beyond the research setting owing to the short half-life of 20 min.
10.3 Diagnosis
Def initive diagnosis has traditionally been the realm of histopathology, but as PET imaging evolves with specific radiotracers including SSTR PET/CT, one can consider it a form of “imaging histopathology,” in some cases obviating the need for invasive biopsy. In the era of multi-slice CT and MRI being more widely performed, incidental neuroendocrine tumors in the pancreas, small bowel, and lung are being identified. These may have anatomic features that favor neuroendocrine etiology such desmoplastic changing surrounding mesenteric lymphadenopathy or arterial enhancement of the primary, but these features are not sufficiently specific to establish a diagnosis. While biopsy remains the first-line diagnostic choice, this cannot singularly be regarding as a gold standard as it is subject to sampling error. There is also morbidity associated with endoscopic or percutaneous biopsy, and depending on patient comorbidities and anatomic location, it may not be feasible. We now utilize SSTR PET/CT to confirm the diagnosis of NET in selected patients. If very intense uptake is demonstrated at site anatomically consistent with a neuroendocrine tumor, diagnosis can be established with a high degree of certainty on imaging alone.
Patients presenting with symptoms due to hormone secretion and elevation of specific hormones and clinical symptoms are another group where SSTR PET/CT is useful to localize the primary site of disease and confirm the diagnosis. This includes patients with carcinoid tumors, insulinoma, glucagonoma, gastrinoma, and VIPoma. We now utilize SSTR PET/CT as the first-line imaging investigation as CT and MRI have a relatively low yield and specificity (see Fig. 10.3). The use of SSTR PET/CT may also obviate the need for endoscopic assessment or direct the endoscopy in cases where histopathologic correlation is required. While most well-differentiated NETs have high SSTR expression making SSTR PET/CT the investigation of choice, this is not universally true. In particular, a proportion of insulinomas do not have SSTR expression. In these patients, we perform 68Ga-Exendin-4 PET/CT which targets the glucagon-like peptide-1 receptor (GLP-1) which is highly expressed in SSTR-negative insulinomas [6, 7].
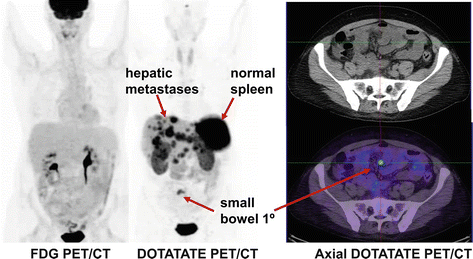

Fig. 10.3
Middle-aged man who presented with carcinoid syndrome, confirmed by the biopsy of hepatic metastatic disease which demonstrated well differentiated tumor with Ki-67 less than 1 %. FDG PET/CT shows no abnormal uptake. In the conventional imaging paradigm, this would be considered a false negative, but the absence of uptake is concordant with the biopsy and supports all sites of disease being indolent Grade I neuroendocrine tumor. DOTATATE PET/CT demonstrates intense uptake in the hepatic metastases indicative of high somatostatin cell surface expression and serving as a predictive biomarker of response to SSA therapy. The primary site of disease which is not visualized on conventional imaging is localized with confidence to the small bowel
10.4 Tumor Grade and Tumor Heterogeneity
Establishing the grade of neuroendocrine tumor is pivotal for selecting the appropriate therapy for an individual patient. Molecular imaging complements histopathologic analysis of individual sites by enabling a whole-body assessment of tumor phenotype. High uptake on DOTATATE and low uptake on FDG PET/CT are seen in patients with well-differentiated NETs commensurate with the high SSTR expression and low proliferative rate. The converse is seen in poorly differentiated tumors. In patients with advanced disease and particular in patients with intermediate or high-grade neuroendocrine tumors, we utilize molecular imaging to guide biopsy as tumor heterogeneity is frequently observed (see Fig. 10.4). In the conventional paradigm, biopsy typically targets the site that is most accessible. Such blinded biopsy may yield tumor specimen that is not reflective of disease at other sites. This can misguide management, particularly if biopsy demonstrates low-grade disease in a patient with occult high-grade disease at other sites. Such patients may be erroneously reassured that they will follow an indolent course only to unexpectedly progress. We utilize both FDG and SSTR PET/CT to image sites of poorly differentiated and well-differentiated disease, respectively (see Fig. 10.5). Biopsy is targeted at sites of most FDG-avid disease which are likely the more aggressive sites that will determine the patients’ prognosis.
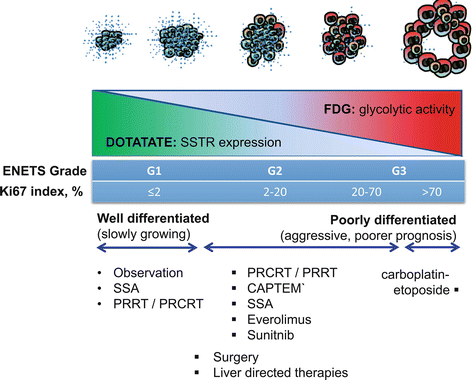
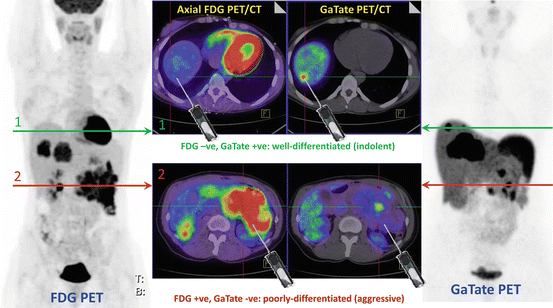

Fig. 10.4
The “flip-flop” phenomenon of high DOTATATE and low FDG uptake in well-differentiated tumors and the opposite imaging phenotype in poorly differentiated tumors. Inter-tumoral heterogeneity may be present with different grade tumors at different sites. Using the prognostic and predictive power of FDG and DOTATATE enables better selection of management for an individual patient. It should be noted, however, that the pattern is not universal as some highly differentiated tumors do not express somatostatin receptors (e.g., insulinomas) and not all aggressive tumors use glycolytic metabolism for growth

Fig. 10.5
Patient with insulinoma and CT/MRI demonstrating a large pancreatic tail primary with locoregional nodal and multiple hepatic metastases. FDG and DOTATATE (GaTate) PET/CT demonstrate different grade tumors at different sites. Without the knowledge of the PET findings, random core biopsy on the basis of CT or MRI could reveal either subtype, which might misinform decision-making. The PET studies demonstrate tumor heterogeneity suggesting both well- and poorly differentiated disease at different sites (Modified with permission from [2])
10.5 Using Molecular Imaging to Guide Management
In the conventional cancer imaging paradigm with anatomic imaging, prognosis can be inferred from the stage of disease determined by the extent of the primary tumor, nodal spread, and distant metastatic disease according to the TNM staging system. In this paradigm, histopathologic features, in particular the proliferative rate measured using Ki-67, are further used to characterize the grade of the tumor. In the molecular imaging paradigm, information from PET/CT is independently valued for establishing the grade and prognosis of the patient which is pivotal in directing therapy, as more aggressive treatment is needed in patients with poorer prognosis, whereas patients with very indolent disease on the other end of the spectrum can sometimes be observed.
In a prospective study of 98 patients with neuroendocrine tumors, 58 % of FDG PET were positive, and in multivariate analysis, SUVmax > 3 was the only predictor of progression-free survival [8] (Fig. 10.6). The study demonstrated that FDG PET/CT provides strong prognostic information which exceeded traditional markers such as Ki-67, chromogranin A, or the presence of hepatic metastases. Other studies have confirmed these results [9, 10]. Conversely, high uptake on SSTR PET indicative of a well-differentiated phenotype confers a favorable prognosis [11]. These studies highlight the ability of whole-body PET imaging to characterize all sites of disease in a given patient, minimizing the sampling error inherent with histopathologic sampling of a random biopsy site. Based on these results, we integrate PET phenotype as a critical factor when stratifying patient risk and deciding on therapy in conjunction with the European Neuroendocrine Tumor Society (ENETS) pathologic grading system [12].
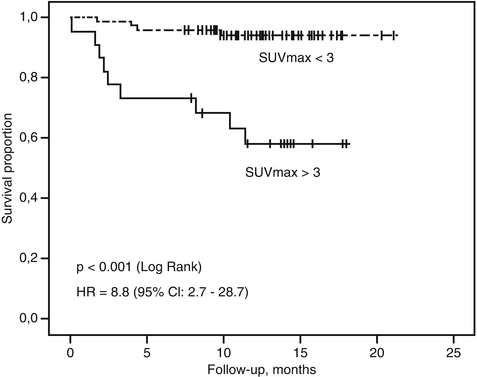

Fig. 10.6
Kaplan–Meier survival curves in a prospective cohort of patients with neuroendocrine tumors. FDG PET provided superior prognostication with overall survival compared to histopathologic Ki-67 (Reproduced with permission from [8])
The improved accuracy obtained when imaging patients with SSTR PET/CT confers a high management impact with an inter-modality change of therapeutic strategy in 19 to 71 % demonstrated in several retrospective studies [13–19]. Most recently, a prospective study of 78 patients demonstrated significant management impact of 36 %, with SSTR PET/CT being equivalent or superior to 111In-octreotide in all patients [20]. In recent years, there are an increasing number of therapeutic options for patients with neuroendocrine tumors. Selecting the optimal therapy or sequence of therapies for an individual patient can be challenging. In our center, molecular imaging staging and characterization play a key role in selection and sequencing of therapy as detailed below.
10.6 Surgery
Surgery remains the cornerstone of curative management of patients with localized neuroendocrine tumors. As detailed above, the use of SSTR PET/CT usually enables confident localization of the primary site which can direct patients to curative surgery, especially those with localized disease. In patients with incidental small well-differentiated tumor discovered on imaging performed for other reasons, observation may be appropriate in a select population. The strongest evidence of such a conservative approach is for incidental well-differentiated pancreatic NET less than 2 cm in size [21]. This may also be true for small incidental lesions in the small bowel and lung. Debulking surgery in patients with metastatic disease can be useful, particularly in patients with symptoms due to secretion of hormones, in whom quality of life can be improved. The role of non-curative debulking surgery is, however, diminishing due to more sensitive imaging with SSTR PET/CT which often demonstrates more extensive disease than conventional anatomic imaging (see Fig. 10.7) and with the availability of new systemic therapeutic options as detailed below. In patients with disease confined to the liver who are being considered for surgery, additional imaging with MRI should be performed to complete staging as this modality can identify small metastases below PET/CT resolution which may modify management.
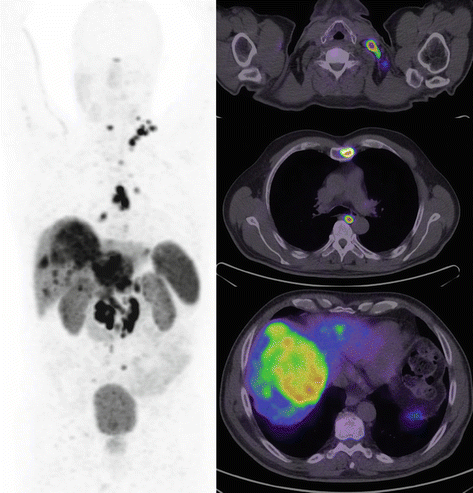

Fig. 10.7
Middle-aged man with metastatic ENETS Grade II pancreatic tumor of unknown primary and documented disease progression despite treatment with somatostatin analogue therapy. Following workup with CT and MRI, the patient was scheduled to undergo hepatic debulking surgery on the basis that more than 90 % of disease could be excised. DOTATATE PET/CT demonstrated additional disease within the bone and lymph nodes on both sides of the diaphragm. In the setting of significant burden of extrahepatic disease not appreciated on conventional imaging with all sites of disease demonstrating high SSTR expression, management was changed to peptide receptor radionuclide therapy (PRRT)
10.7 Somatostatin Analogue (SSA) Therapy
Somatostatin analogues (SSA) such as lanreotide or octreotide LAR are now commonly used to treat patients with unresectable NET. This was originally used for patients with symptoms due to hormonal secretion but is now also used to slow progression in selected patients. A key selection criteria for commencing SSA are adequate SSTR expression in the tumor, which is defined by imaging. In the CLARINET trial [22], this was defined by a Krenning score of Grade II or higher on 111In-octreotide scintigraphy. In this semiquantitative scale [23], there are five scores ranging from 0 to 4 defined as no uptake, very low-intensity uptake, uptake intensity less than or equal to the liver, uptake intensity greater than the liver, and uptake intensity greater than the spleen, respectively. Although this scale was designed for planar scintigraphy, we continue to use this scale for reporting SSTR PET/CT.
10.8 Radionuclide Therapy
Labeling SSA with therapeutic beta emitters enables targeted treatment of non-resectable neuroendocrine tumors. Peptide receptor radionuclide therapy (PRRT) with 177Lu was pioneered by the Erasmus group who demonstrated highly favorable results in a large cohort of patients back in 2005 [24]. Since this time, the treatment has evolved [24–34] with additional use of 90Y-radiolabeled SSA and combining PRRT with chemotherapy, the so-called peptide receptor chemoradionuclide therapy (PRCRT) [27, 35, 36]. Similar to SSA therapy, the use of SSTR scintigraphy or SSTR PET/CT is essential for selection of patients that may benefit from PRRT. Studies to date have required uptake intensity greater than the liver corresponding to a Krenning score of 3 to indicate potential benefit and suitability for PRRT. While SSTR PET/CT is generally superior to SSTR planar or SPECT imaging, there are a minority of patients where early time-point imaging performed with 68Ga has lower intensity uptake compared with more delayed imaging performed with 99mTc or 111In-SSTR SPECT/CT.
We additionally employ FDG PET/CT in selected patients, usually those with ENETS Grade II or higher tumors, to further enrich the selection for patients likely to benefit from PRRT (see Table 10.1). For patients with sites of FDG+/SSTR- disease, we usually do not recommend PRRT as these patients have sites of aggressive NET that cannot be targeted (see Fig. 10.8). This recommendation is not universal as the addition of concomitant chemotherapy with PRCRT may target these sites, while patients with symptoms due to hormonal secretion may still derive symptomatic benefit by treating the well-differentiated disease components (see Fig. 10.9).
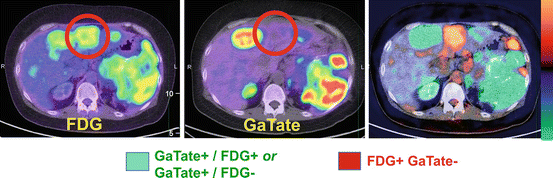
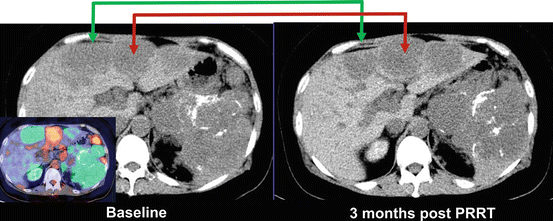
Table 10.1
Our recommendation for indications of selective use FDG PET/CT in addition to SSTR PET/CT
Ki-67 ≥ 5 % |
Lesions of concern on the CT component of SSTR PET/CT with low or no activity |
Clinical or radiologic evidence of disease progression within a time frame less than 6 months despite a Ki-67 < 5 % |

Fig. 10.8
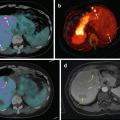
This patient with metastatic neuroendocrine tumor and known hepatic metastases was evaluated for suitability for peptide receptor radionuclide therapy (PRRT). FDG and DOTATATE PET/CT demonstrated several sites of discordant FDG+ DOTATATE- (orange/red color) disease, although the majority sites were DOTATATE+ FDG+ or DOTATATE+ FDG- (green color). This patient is not an ideal candidate for PRRT as discordant FDG+ sites will not be targeted with PRRT but represent the sites of most aggressive disease that more likely determine patient prognosis and outcome

Fig. 10.9
The patient in Fig. 10.8 had intractable hypercalcemia due to PTHrP secretion from the neuroendocrine tumor. In view of likely hormone secretion from the well-differentiated disease, the patient was treated with PRRT [37] despite the presence of FDG+ DOTATATE- sites of disease. As predicted by the baseline molecular imaging phenotype, sites of DOTATATE+ disease (whether FDG+ or FDG-) responded to PRRT (green arrow), but sites of discordant FDG+ disease progressed (red arrow)
Our application of PRRT and PRCRT is highly individualized [38]. In general, for patients with ENETS Grade I tumor, we only use radionuclide therapy for patients who, despite SSA therapy, have objective evidence of disease progression or have ongoing symptoms. For patients with more aggressive ENETS Grade II or higher tumors, we now routinely use PRCRT as the first-line treatment [39] given the significantly greater response rates and survival rates seen with the individualized theranostic approach compared to other therapeutic options [40]. For patients with tumors greater than 4 cm in size, we generally administer one or two cycles with 90Y-DOTATATE followed by further cycles of 177Lu-DOTATATE up to a total of four cycles. For patients with very large burdens of disease, we consider early consolidation with one to two further cycles of PRRT. We individualize the administered activity according to tumor burden seen on SSTR PET/CT, body weight, and renal function [38].
The adverse prognosis associated with FDG-avid NET as described above was described in the pre-PRRT era. We have recently published data regarding the efficacy of peptide receptor chemoradionuclide therapy (PRCRT) with Lutetium-177 DOTATATE (LuTate) combined with 5-fluorouracil (5FU) in a cohort of 52 patients with FDG-avid NET [41]. Despite the anticipated poor prognosis of this cohort, we demonstrated an unexpectedly long progression-free survival (PFS) of 48 months, with median overall survival of 55 months [40]. While retrospectively, our results have a lead-time bias which is disadvantageous to our analysis, as survival was measured not from diagnosis but from the time of PRCRT in a population who were previously treated with conventional therapeutic regimens including at least one line of chemotherapy in 67 %. We believe these favorable results demonstrate how PRCRT can successfully treat sites of poorly differentiated disease, “converting” some patients with aggressive NET back to a more indolent phenotype.
Post-therapy imaging is routinely performed which enables visualization of the actual radiotracer delivery. Dosimetry can be performed using a variety of techniques but is beyond the scope of this chapter. Our preference is to perform quantitative SPECT/CT for 177Lu [42] or PET/CT for 90Y, followed by voxel-based lesional dosimetry [43]. Knowledge of the actual dose of radiation delivered to sites of tumor and normal tissues is informative, but it can be challenging to use this information to inform future therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree