Multiple endocrine neoplasia type 1 (MEN 1)
Multiple endocrine neoplasia type 2 (MEN 2)
Familial hypocalciuric hypercalcemia
Familial hypercalciuric hypercalcemia
Hyperparathyroidism—jaw tumor syndrome
Familial isolated hyperparathyroidism
MEN1 can be considered a rare cause of PHPT, the incidence of MEN1 being about 2–4 % of PHPT cases. However, PHPT is the most common endocrinopathy in the MEN1 syndrome: it is found in almost 100 % of the patients over 50 years of age and constitutes the first sign of the disease in most carriers in their twenties [5]. The diagnosis of PHPT in young adults should therefore include the search for MEN1. The search for MEN1 should also be conducted in the immediate family. The prevalence of PHPT in MEN-2 is lower than in MEN-1 and is found in 20–30 % of cases. Furthermore, the majority of patients with PHPT present clinical manifestations that are more discrete than the clinical signs demonstrated by MEN-1 carriers [6].
The jaw tumor hyperparathyroidism syndrome is a rare disease. Evidence shows that bone tumors of the jaw related to PHPT can be outlined [7]. Parathyroid cancer has been also detected in more than 15 % of cases [7].
Diagnosis
In familial isolated hyperparathyroidism, cases of PHPT are diagnosed in the immediate family in the absence of other endocrinopathies; this characterizes the phenotype as a hidden syndrome, such as MEN-1 and MEN-2 [4].
Hypercalcemia and an increase in PTH levels constitute the biochemical markers of PHPT. In normal conditions of hypercalcemia there is an inhibition of the parathyroid glands. This inhibition is translated by low levels of PTH [3].
The majority of patients with PHPT present slightly increased levels of PTH, but up to 10 % of the cases can exhibit normal to upper normal levels of PTH [8, 9]. Nevertheless, these levels are inappropriately high because of the hypercalcemia. Hypercalcemia is found in most of the cases of PHPT, but one has to consider the possibility of fluctuations in the levels of serum calcium, which could explain the normal levels of calcemia [8].
Diagnosis includes the evaluation of serum concentrations for calcium, phosphorus, albumin, alkaline phosphatase, intact PTH, 25 (OH)-D vitamin, and renal function [8, 9]. 24-h urinary calcium and the serum levels of creatinine must be evaluated in order to rule out the possibility of familial hypocalciuric hypercalcemia [10, 11].
About 40 % of serum calcium is linked to albumin. Serum levels must be adjusted according to the following equation: corrected calcium = serum calcium (mg/dL) + [0.8 × (4 − serum albumin)]. The measurement of the ionized calcium can be useful in specific cases, such as subjects with hyperalbuminemia, thrombocytosis, Waldenstrom’s macroglobulinemia, and myeloma, since in these subjects there is hypercalcemia with normal ionized serum calcium (artifactual hypercalcemia) [10, 12].
In a retrospective cohort with 6,982 subjects, the serum ionized and total calcium were compared in patients with hypercalcemia. PTH-dependent hypercalcemia was set as total serum calcium (corrected by albumin) equal to or greater than 10.2 mg/dL or ionized calcium (technique of specific ion electrode) equal to or greater than 1.32 mmol/L with the PTH >5pmol/L (35 pg/mL; reference values 0.9–9 pmol/L). Among these subjects, 343 had high ionized calcium and 156 (45 %) had high total calcium. In a second cohort with 203 subjects, 143 presented histologically confirmed PHPT: high ionized serum calcium was present in 141 cases (98 %) and, lastly, increased total serum calcium in 108 (76 %), demonstrating a greater diagnostic accuracy when ionized serum calcium was used [13].
A cohort study, based on the population of Tayside in Scotland, used the following criteria to diagnose primary hyperparathyroidism: (1) serum calcium corrected for albumin >10.22 mg/dL (reference values: 8.4–10.22 mg/dL) on at least two occasions, with serum PTH >13.5 ng/L (reference values: 4.5–31.05 ng/L), or (2) serum calcium corrected for serum albumin >10.22 mg/dL on one occasion with serum PTH >31.05 ng/L [14]. These values of serum PTH correspond to 20 pg/mL for assays with reference values between 10 and 65 pg/mL.
The causes of secondary hyperparathyroidism that can increase the serum levels of parathormone, such as the use of thiazide diuretics [9] and lithium, deficiency of vitamin D, and the use of bisphosphonates, must be excluded. Concerning tertiary hyperparathyroidism due to renal failure, genetic causes such as familial hypocalciuric hypercalcemia also need to be sought. The finding of normal levels for calcium corrected by albumin and associated with high serum PTH in the absence of other causes is compatible with normocalcemic PHPT.
Serum PTH and the biochemical markers of the bone remodeling are significantly higher in patients with severe disease. These patients frequently have vitamin D deficiency and easier localization of the parathyroid lesion than asymptomatic patients [2].
The levels of serum phosphorus are usually found to be in the lower normal range. Specific markers of bone modeling (osteocalcin and alkaline phosphatase osteo-specific) or markers of bone resorption (deoxypyridinoline, N-telopeptide, and C-telopeptide) seem to remain either in the high-normal range or slightly above reference values. Hypercalciuria is found in around 30 % of asymptomatic patients, in 50 % of patients with active urolithiasis, and in 40 % with osteitis fibrosa cystica [2, 9]. Patients with severe PHPT have moderate levels of serum calcium and lower levels of serum phosphorus as compared to asymptomatic subjects (14.0 ± 0.7 vs. 10.9 ± 0.4 mg/dL; p < 0.001 and 2.0 ± 05 vs. 296 ± 0.2 mg/dL; p < 0.01, respectively). In the serum levels of intact PTH, there are greater differences: 1,820 ± 349 vs. 133 ± 29 pg/mL; p < 0.001 [15].
Serum PTH and the biochemical biomarkers of the bone remodeling are significantly higher in the patients with tevere disease, who frequently present vitamin D deficiency and easier localization of the parathyroid lesion than asymptomatic patients [16].
Differential Diagnosis
PHPT needs to be differentiated from other causes of hypercalcemia (Table 22.2), as well as from diseases that can cause osteoporosis, nephrolithiasis, nefrocalcinosis, and hypophosphatemia. PHPT and neoplasia correspond to 90 % of hypercalcemia cases. Data from the literature shows that 50–60 % of outpatients with hypercalcemia are carriers of the PHPT and about 31 % present neoplasia [18].
Table 22.2
Differential diagnosis of hypercalcemia
1) Malignancies | Solid tumors | Solid tumors | Hematologic malignancies (lymphoma, leukemia, multiple myeloma) | Ectopic production of PTH (thyroid carcinoma, ovarian, lung oat cells) |
Osteolytic hypercalcemia (breast, lung) | ||||
Humoral hypercalcemia (carcinomas) | ||||
Squamous lung, esophagus | ||||
2) PTH dependent | NEM | PHPT: | Familial hypercalciuric hypercalcemia (FHH) | Treatment with lithium |
a) Adenoma | ||||
b) Carcinoma | ||||
c) Hyperplasia | ||||
3) Related to vitamin D | Idiopathic familial hypercalcemia | Granulomatous diseases | Vitamin D intoxication | – |
4) Other causes | Milk-alkali syndrome, aluminum intoxication | Endocrine diseases (hyperthyroidism, pheochromocytoma, adrenal insufficiency) | Advanced chronic disease of the liver/kidney | Drugs: |
Thiazide | ||||
Theophylline | ||||
Beryllium |
Hypercalcemia with very low or undetectable PTH plasma levels can be found when the disease is malignant and, in this case, the PTHrp is responsible for the increase in calcium [12]. Several laboratory characteristics associated with malignity are similar to those of PHPT, such as hypercalcemia, hypophosphatemia, hypercalciuria, and hyperphosphaturia and an increase in the nephrogenic cyclic AMP [19]. However, the difference between primary HPT and the malignant disease with hypercalcemia can be identified without difficulty, based on the clinical history of the patient. The hypercalcemia symptoms of PHPT are manifested over months or years, while in malignancy these symptoms are manifested within weeks and are secondary to the underlying malignant disease. Thus, hypercalcemia in malignant disease is readily revealed and is frequently associated with a survival of about 6 months. Other related symptoms are anemia and weight loss. In general, when hypercalcemia is found, malignancy is clinically revealed by imaging techniques or bone metastasis presented by the patient. In addition to these parameters, persistent hypercalcemia of early onset suggests malignancy, whilst a mild hypercalcemia lasting for more than 6 months is more likely to be caused by PHPT.
The definitive differential diagnosis is performed by means of serum PTH measurement. In PHPT, when PTH is increased or within normality the condition may be regarded as PTH-dependent hypercalcemia, but is frequently suppressed in malignant disease, which is independent of PTH [12]. In rare cases, PTH can be increased in malignancy due to ectopic production or when parathyroid carcinoma is the cause of the hypercalcemia [20].
Another differential diagnosis that should always be demanded is that of familial hypocalciuric hypercalcemia, which is characterized by a genetic defect in the calcium receptors in the parathyroid glands and kidneys, inherited as a dominant autosomal disorder [5]. The hypercalcemia is mild and followed by hyperphosphatemia, and levels of PTH are normal or slightly increased. The most pronounced laboratory finding is hypocalciuria, which suggests increased tubular resorption of calcium. This diagnosis is considered in young asymptomatic patients that present (1) levels of serum calcium with a slight-to-moderate increase, (2) hypocalciuria, (3) a familial history of hypercalcemia, and (4) a rate of calcium/creatinine clearance of less than 0.01 [17].
Normocalcemic Hyperparathyroidism
Patients that undergo routine evaluations during an investigation for bone loss may have increased levels of PTH, even without hypercalcemia [21].
The term normocalcemic primary hyperparathyroidism (NPHPT) was first used by Wills in 1960, who described a group of patients having characteristics different to those diagnosed with classic PHPT [22].
NPHPT is characterized by levels of serum calcium that remain normal while PTH levels are high [23–25]. Since there is a greater availability and utilization of assays for the evaluation of this hormone, this condition has been frequently diagnosed. However, examining for other causes of secondary hyperparathyroidism, especially 25-hydroxyvitamin D deficiency, is necessary to confirm the diagnosis [8, 25].
Little is known about the epidemiology of NPHPT. In Sweden, Lundgren et al. studied 5,202 postmenopausal women aged 55–75 years. In the 109 subjects studied, the researchers investigated two indices, observing whether the patients presented hypercalcemia associated with increased levels of PTH and higher levels of either hypercalcemia or PTH. Seventeen (16 %) out of the 109 subjects studied had normal levels of serum calcium (<9.9 mg/dL) and increased PTH. This group of 17 subjects included people that had vitamin D deficiency as well as patients with NPHPT [26].
It remains debatable whether NPHPT incipiently represents classic PHPT or a different spectrum of this pathology [24]. Evidence suggests that patients without secondary causes of hyperparathyroidism may have early-stage PHPT since, if the disease is diagnosed early, it can progress with isolated increased serum PTH, which may or may not be followed by an increase in serum calcium. For these patients, serum calcium should be periodically evaluated during the development of the disease [25, 27].
Skeletal Manifestations
The skeletal complications of PHPT are well known. Among the classic symptoms, these complications are considered the most familiar consequences of PHPT. The clinical presentation may include focal or widespread bone pain, localized bone edema (“brown tumors”), and fragility fractures [21].
Intense bone demineralization is seen in X-rays of patients with this severe disease. Pathological fractures are frequently seen, especially in the long bones of the lower extremity, and also loss of the lamina dura of the teeth and brain lesions in the salt-and-pepper pattern which refers to the speckled appearance of the tissue. Subperiosteal bone erosions in the distal phalanges and on the edges of the medial phalanges are usually seen as numerous lytic lesions with irregular sclerotic margins, which are more common in the pelvis, long bones, and shoulders. The cortical bone of the long bones is extremely thin and in some patients is almost absent [16].
Bone densitometry is a useful tool for investigating the classic effects of PTH, such as reduction in bone mineral density (BMD) in the distal radius, the site of the cortical bone. The catabolic ability of PTH on the cortical bone is the opposite of its anabolic effect on cancellous bone. In the lumbar spine, the site of cancellous bone, BMD seems to be normal. The hip contains a more uniform mix of cortical and cancellous bone elements and the BMD is classified as being of an intermediate density between the distal radius and the lumbar spine. Although this classic densitometric profile is usually seen as a distinct pattern characterized by vertebral osteopenia, it can also be seen at the moment of diagnosis. In the more severe types of PHPT, there is an overall decrease in bone density [28].
The prevalence of PHPT and its impact on BMD were evaluated in 3,014 men aged 69–81 years in a Swedish cohort, MrOs. Subjects with a low glomerular filtration rate (<21 mL/min/1.73 m2) and vitamin D deficiency (<50 nmol/l) were excluded from the study. BMD was compared between patients with and without PHPT. The prevalence of PHPT was estimated to be 0.73 %. BMD in the total hip and femur neck was lower among the PHPT group than in the control group. Subjects with high levels of intact PTH were compared with the other subjects from the cohort. For that subgroup, BMD was lower for the total hip and lumbar spine (p <0.05) [29].
A controlled clinical trial compared two groups: (1) carriers of mild PHPT that were submitted to parathyroidectomy (n = 25) and (2) patients that had an intact parathyroid (n = 28). After 24 months, there was a significant increase in the BMD in the femur neck and total hip, but not in the lumbar spine or forearm of patients submitted to parathyroidectomy when compared with those that did not undergo a parathyroidectomy. There was also a decrease in the biochemical markers of bone remodeling after parathyroidectomy [30]. Another study with 11 patients, including a 5-year follow-up after parathyroidectomy, showed a significant BMD increase in the lumbar spine. However, neither the hip nor the distal radius showed any BMD increase when compared to baseline values. They also observed a reduction in the markers for bone remodeling [31].
Extraskeletal Manifestations
Neuropsychiatric Symptoms
In addition to skeletal manifestations, PHPT may be associated with alterations in other organ systems within the body. Neuropsychiatric symptoms can occur in about 23 % of patients with PHPT, such as fatigue, difficulty concentrating, irritability, and mood and sleep disorders [32]. Since few studies have evaluated the prevalence of these manifestations, they remain uncertain [33]. A case–control study compared 39 postmenopausal patients with mild PHPT and 89 women without PHPT. This study revealed a higher prevalence of depression and anxiety and a higher performance on tests for verbal and nonverbal memory in the PHPT carriers. Also observed was the fact that depressive symptoms, nonverbal abstraction, and aspects of the verbal memory were significantly improved after parathyroidectomy [34]. Peripheral neurological alterations, especially sensory–motor polyneuropathy and PHPT, have been suggested by some authors [35, 36]. Recent data report clinical improvement after surgical treatment, which is recommended in patients that have neurological symptoms related to PHPT and do not present any contraindication for surgery [37].
Cardiovascular Symptoms
The literature shows a relationship between PHPT and abnormalities such as arterial hypertension, left ventricular hypertrophy, abnormal heart function, coronary artery disease, vascular abnormalities, conduction disorders, and valvular and myocardial calcification [38]. The mechanism for this aforementioned relationship remains uncertain, but it has been shown that morbidity and the risk of cardiovascular death are greater in PHPT carriers. This is mainly observed in patients with the mild to severe form of the disease [39]. On the other hand, parathyroidectomy decreases cardiovascular risk, as shown in a number of population studies, even with mild forms of the disease. Surgery is therefore indicated in all patients with PHPT and other factors of cardiovascular risk [40, 41].
Other Extraskeletal Manifestations
Nephrocalcinosis and nephrolithiasis are also clinical manifestations of asymptomatic and NPHPT. From the experience of our group, a recent study demonstrated an 18.2 % rate of nephrolithiasis in NPHPT patients, as well as in the hypercalcemic modality (18.9 %), may have show a non-indolent presentation [42].
Localization of Parathyroid Lesions
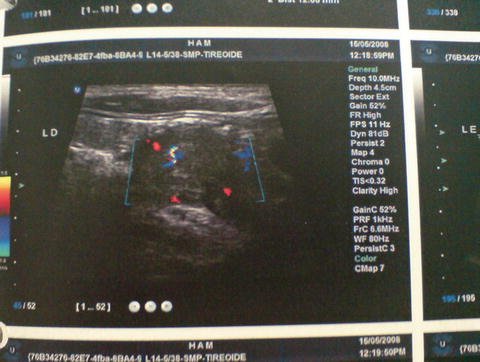
Imaging examinations are not indicated for the diagnosis of PHPT. The location of the affected parathyroid is an indication for surgery and can permit the use of less invasive techniques, which is associated with a lower morbidity rate [43]. Ultrasonography and Sestamibi scintigraphy are the most common techniques used for PHPT diagnosis (Figs. 22.1 and 22.2). Cervical ultrasonography is a low-cost examination, as well as noninvasive. When performed by an experienced examiner, it presents a sensitivity and specificity of 88 and 94 %, respectively [44]. In cases of ectopic glands or an intrathyroid adenoma, identification and differentiation of thyroid nodules can be difficult. Thus, ultrasonography coupled with 99mTc-labeled Sestamibi scintigraphy increases the chance of identification to almost 100 % of the lesions [45]. These two methods are complementary, since ultrasonography provides anatomic information while the scintigraphy provides functionality data.
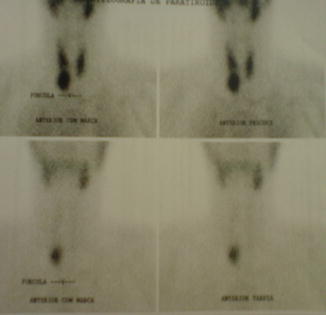

Fig. 22.1
Tc-99-Sestamibi scintigraphy showing a right inferior parathyroid lesion
Scintigraphy is able to identify the topic and ectopic parathyroid tissues. A study with 64 PHPT patients presented positive scintigraphy in 64 % of the patients that had asymptomatic PHPT and 83 % of the group that carried nephrolithiasis without bone involvement. That same study showed that 100 % of the subjects with the severe disease presented positive scintigraphy as well, but in this case it was characterized by osteitis fibrosa cystica. These results were found when the imaging was evaluated early, which occurred in 70 % of the cases analyzed [45] (Fig. 22.3). A small number of patients may have negative imaging, which suggests multiglandular disease. In these cases, the use of the most advanced imaging techniques may be necessary to increase the chances of localizing the affected parathyroid and ectopic tumors, and also assist in the decision to proceed with surgery. Four-dimensional computed tomography was able to localize the adenoma with 82 and 92 % sensitivity and specificity, respectively, in 34 PHPT patients [46]. A retrospective trial found almost 100 % specificity in the diagnosis of multiglandular disease in 35 patients evaluated; however the sensibility was much lower (42.9 %). As regards the localization of only one lesion, they were able to identify 32 cases with a sensibility of 91 % [47]. The preoperative localization of an adenoma allows the use of minimally invasive parathyroidectomy (MIP) with lower morbidity and can be done in an outpatient facility [47].
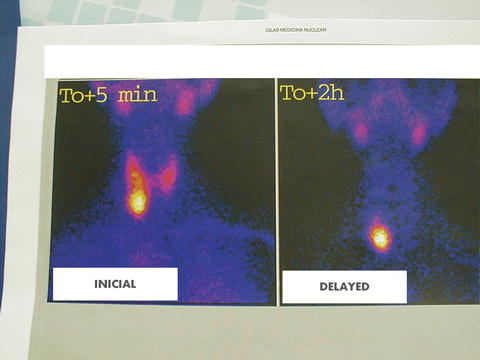

Fig. 22.3
99mTc-Sestamibi scintigraphy showing a large parathyroid adenoma
One of the main disadvantages of imaging examinations is the high incidence of false-positive results due to the size and localization of the parathyroid affected. As a result, fine needle aspiration of the nodule that was identified by imaging for measurement of PTH in the aspirated material has become an auxiliary method for diagnosing lesions, as shown by a study performed by our group. A group of 15 women without PHPT, who had nodules identified by ultrasonography, showed very high PTH levels, presenting a mean of 4.919 ± 5.124 pg/mL whilst the control group had a mean of 10.65 ± 3.49 pg/mL. This technique showed a greater sensitivity in locating the affected gland than the use of imaging alone [48]. The main methods for locating parathyroid lesions are shown in Table 22.3.
Table 22.3




Localization procedures for the identification of parathyroid adenoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree