37 Primary Central Nervous System Lymphoma
Primary central nervous system lymphoma (PCNSL) is a rare aggressive non-Hodgkin lymphoma (NHL) confined to the craniospinal axis including the brain parenchyma, leptomeninges, eyes, or spinal cord. It is recognized as a discrete entity in the 2008 World Health Organization classification.1 It represents about 1% of all NHLs and 2 to 5% of all primary intracranial tumors. Men and women are equally affected, with an annual incidence rate of 0.47 per 100,000 person-years and a median age of 60.2 PCNSL is associated with congenital or acquired immunodeficiency, particularly with human immunodeficiency virus (HIV), but the incidence in immunocompetent patients has risen over the past three to four decades.3
 Biology and Pathogenesis
Biology and Pathogenesis
Primary central nervous system lymphoma is restricted to the central nervous system (CNS), despite there being no lymphoid tissue in the nervous system. About 90% of PCNSLs are diffuse large B-cell lymphomas (DLBCLs). Only a small proportion have Burkitt (5%), lymphoblastic (5%), marginal zone (3%), or T-cell lymphoma histotype (2–3%). PCNSL typically has an angiocentric growth pattern and phenotypically expresses pan–B-cell markers (CD20, CD19, CD22, CD79a). Approximately 80% represent nongerminal center lymphomas, and only about 20% have a germinal center phenotype; all are negative for Epstein-Barr virus (EBV), unlike PCNSL in immunocompromised patients, which is characteristically EBV-driven. The proliferating index is usually 50 to 90%.4
 Clinical Presentation
Clinical Presentation
At presentation, cerebral symptoms are the most common, followed by ocular, leptomeningeal, and spinal cord symptoms (Table 37.1).
Seizures occur less frequently (10%) in PCNSL than in gliomas or metastatic lesions (25–35%). Symptom duration prior to definitive diagnosis averages 1 to 3 months, reflecting its rapid growth rate.
Ocular involvement is seen in approximately 20% of PCNSL patients and may be unilateral or bilateral. Ocular symptoms typically include floaters and blurred or diminished vision, but many patients have no visual symptoms, and ocular involvement is detected only on slit-lamp examination. About 15% of patients have a positive cerebrospinal fluid (CSF) cytology, although focal leptomeningeal involvement can be identified in almost all patients at autopsy.
Table 37.1 Symptoms of Primary Central Nervous System Lymphoma
| Cranial |
| Personality/cognitive changes |
| Lateralized: e.g., hemiparesis, aphasia |
| Seizures |
| Headache |
| Cranial neuropathy |
| Ocular |
| Floaters |
| Blurred or cloudy vision |
| Diminished visual acuity |
| Spinal |
| Back pain |
| Radiculopathy |
| Limb weakness |
| Sensory level or paresthesias |
| Bowel or bladder dysfunction |
Pearl
• When systemic NHL metastasizes to the CNS, it usually involves the leptomeninges and only rarely the brain parenchyma, whereas PCNSL primarily involves the brain.
 Diagnostic Procedures
Diagnostic Procedures
Imaging
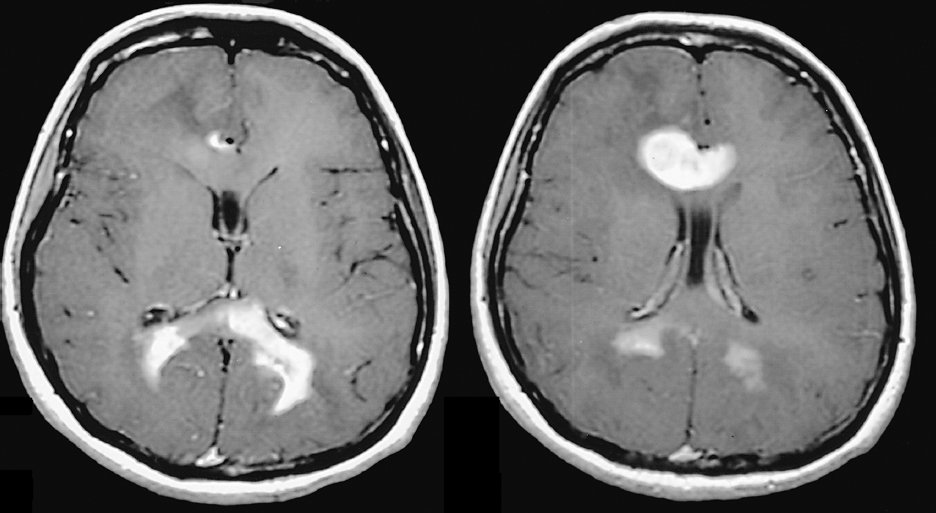
Magnetic resonance imaging (MRI) is the preferred imaging modality, unless contraindicated, to identify brain or spinal cord PCNSL, which is typically hypointense to isointense on T1-weighted images, with intense homogeneous contrast enhancement (Fig. 37.1). Nonenhancing lesions can occur, and peritumoral edema is often less than expected. Acquired immunodeficiency syndrome (AIDS)-related PCNSL lesions are often ring-enhancing on T1-weighted images and may be associated with hemorrhage or necrosis. PCNSL lesions are typically supratentorial, periventricular, and involve deep structures such as the basal ganglia. They are single in approximately 60% of patients and multiple in 40% (Fig. 37.1); more than 90% of AIDS patients have multiple lesions. Unlike other primary brain tumors, PCNSL is usually bright on diffusion sequences with corresponding hypointensity on apparent diffusion coefficient (ADC) sequences. Single photon emission computed tomography (SPECT) scanning using gallium 67 and thallium 201, positron emission tomography (PET), and methionine PET all show increased uptake in PCNSL tumors.
Pitfall
• Steroid response should not be used intentionally as a diagnostic test for PCNSL because other CNS processes, such as multiple sclerosis or sarcoidosis, can have a similar imaging appearance and response to steroids.
Biomarkers
In a multicenter retrospective series, CXC chemokine ligand (CXCL)13 and interleukin-10 (IL-10) in the CSF were identified as a complementary biomarker pair that yields diagnostic information in most PCNSL cases, with sensitivity significantly greater than with standard CSF tests, cytology, and flow cytometry, and equivalent to the sensitivity of results obtained from brain biopsy.5 More recently, CXCL13 alone was found to discriminate greater than 70% of PCNSL cases with 94.9% specificity; diagnostic sensitivity can be improved to 84% by parallel evaluation of IL-10, which also provides prognostic information while maintaining greater than 90% specificity.6 Although the precision of the cutoff points for maximal diagnostic accuracy need to be defined prospectively, CXCL13 and IL-10 may be used to guide the workup of potential PCNSL in patients for whom brain biopsy may be of heightened risk or low diagnostic yield.
 Staging and Pretreatment Investigations
Staging and Pretreatment Investigations
Primary central nervous system lymphoma is classified as a stage IE NHL because it is restricted to a single extranodal site.
Systemic workup identifies extraneural disease in approximately 6% of patients, and all sites can be identified by abdominal and pelvic computed tomography (CT) scan or bone marrow biopsy, suggesting that, if performed, systemic staging can be limited to these tests.7 In all patients, outcome is determined by the CNS disease and not by the systemic lymphoma. More importantly, staging of the nervous system must be thorough (Table 37.2).
 Prognosis
Prognosis
Two main prognostic scoring systems have been developed. In the International Extranodal Lymphoma Study Group (IELSG), age older than 60 years, Eastern Cooperative Oncology Group (ECOG) performance status greater than 1, elevated serum lactate dehydrogenase, high CSF protein concentration, and involvement of deep brain regions were all independently associated with worse survival.8 The Memorial Sloan-Kettering Cancer Center group established and validated a simpler and widely applicable model with three risk groups: age ≤ 50 years (low); age > 50 years and Karnofsky Performance Scale (KPS) score ≥ 70 (intermediate); and age > 50 years and KPS < 70 (high).9
Table 37.2 Essential Studies for Staging Disease in Patients with Primary Central Nervous System Lymphoma
| Contrast-enhanced imaging of the brain, preferably MRI |
| Ophthalmologic evaluation (including slit-lamp examination) |
| Lumbar puncture |
| Human immunodeficiency virus test |
| Body CT scan ± body PET |
| Contrast-enhanced MRI of the spine (if clinically indicated) |
There are likely pathological prognostic factors in PCNSL similar to what has been identified in systemic DLBCL.10 In systemic NHL, germinal center phenotype, defined by either gene expression profiling or a pattern of cell surface marker expression by immunohistochemistry (IHC), is associated with better survival. Most such reports on PCNSL also suggest that germinal center phenotype predicts better outcome, but this is not universal and needs verification in larger cohorts.
Imaging may help predict the clinical course of patients. Early complete response (CR) assessed by MRI indicated long-term survival in a cohort of 88 patients treated with a polychemotherapy regimen. A significantly longer survival was observed for those patients who achieved complete remission after two treatment cycles compared with those who reached CR after six cycles.11
 Treatment
Treatment
Primary central nervous system lymphoma is sensitive to chemotherapy and radiotherapy, so that durable complete remissions are possible. However, the outcome is still unsatisfactory when compared to other NHLs. There is consensus that high-dose methotrexate (HD-MTX) is the single most important chemotherapeutic agent, but there is no standardized combination regimen.
Current therapeutic knowledge in PCNSL is based primarily on single-arm phase 2 studies; only one randomized phase 2 trial12 and one phase 3 trial13 have been reported. Besides the rarity of the disease, the success of clinical trials is limited by the fact that many patients present with severe symptoms and various comorbidities, compromising enrollment. Thus, the level of evidence for therapeutic choices in PCNSL remains low.
Surgery
Gross total resection is often not possible due to the deep location and multifocality of most PCNSL lesions. Most reports suggest there is no survival advantage to gross total resection, but a recent report suggests that debulking may improve outcome.14,15 Furthermore, tumor debulking may be indicated for urgent decompression in a patient with neurologic deterioration because of mass effect. However, in most patients, image-guided/stereotactic biopsy is the preferred method for obtaining a tissue diagnosis in patients suspected of having PCNSL.
Pitfall
• Administration of glucocorticoids prior to performing a biopsy may lead to a nondiagnostic specimen and hinder establishing the correct diagnosis. Corticosteroids are oncolytic in PCNSL, identical to their activity against systemic NHL. The initial approach to patients with lesions consistent with PCNSL is to withhold steroids prior to biopsy unless the patient is decompensating clinically. The lympholytic effect of steroids can be seen in CSF and vitreal specimens as well.
Radiotherapy
Primary central nervous system lymphoma is a radiosensitive tumor, and radiotherapy (RT) was the first treatment modality to prolong median survival to between 12 and 18 months,16 a marked increase from the 2- to 3-month survival of untreated patients. Whole-brain radiotherapy (WBRT) is recommended because of the diffuse, infiltrative, and often multifocal nature of PCNSL. Doses in the range of 40 to 50 Gy are used, with single fractions of 2 Gy or less. Higher doses or the addition of a boost does not enhance disease control, but is associated with increased neurotoxicity.17
Radiotherapy in Combination with Chemotherapy
To increase remission rates and prolong overall survival (OS), the first structured efforts to use systemic chemotherapy combined it with WBRT. Better response rates and improved survival were reported in clinical trials using HD-MTX–based chemotherapy followed by WBRT. Complete response rates ranged between 69% and 87% using combined chemo-radio-therapy, with a median progression-free survival (PFS) of 24 to 40 months.18,19 This resulted in widespread use of combined modality treatment in the primary management of PCNSL. Unfortunately, with longer follow-up, severe neurologic impairment developed after combined treatment particularly in older patients.20 This led to the investigation of RT dose reduction and RT omission in patients with a CR after chemo-therapy alone. Neurotoxicity risks are age-related and the risk rises with increasing age.
The only randomized control trial (G-PCNSL-SG) completed in PCNSL examined the role of WBRT in combination with MTX-based therapy.13 All patients received six cycles of single-agent MTX, 4 g/m2, ± ifosfamide (1.5 g/m2) in 14-day cycles. Patients who achieved a CR were randomized to be followed or to receive 45 Gy WBRT. A total of 551 patients were enrolled, but only 58% completed the study as per the protocol. In all patients, WBRT prolonged PFS from 12 to 18 months (p = 0.041 in the intent to treat [ITT] population), but OS was similar at 37 versus 34 months (0.94, ITT population). The CR rate following chemotherapy was 35% overall and was similar in patients younger and older than 60 years of age. However, the overall response rate (CR + partial remission) was better in younger patients (63% vs 49%). Although remarkable for its size, the study was limited by high dropout rates, variable chemotherapy, underpowered noninferiority design, and insufficient neurotoxicity evaluation. These data suggest that WBRT does not prolong survival and, therefore, can be eliminated to reduce neurotoxicity. However, the G-PCNSL-SG trial did not really study neurotoxicity, and the potentially negative impact of early relapse and salvage therapy on cognitive function has never been evaluated.
As an alternative strategy, reduced-dose WBRT has been examined in a series of studies that suggest that it retains efficacy in patients with a response to chemotherapy, and there are various reports on its risk of neurotoxicity. In an early study,21 reducing the dose of WBRT to 30.6 Gy compromised survival in patients younger than but not older than 60 years. In Radiation Therapy Oncology Group (RTOG) trial 9310, decreasing WBRT from 45 to 36 Gy in patients achieving a CR to chemotherapy did not compromise survival but also did not reduce the frequency of neurotoxicity, although onset was delayed. More recently, in a phase 2 trial, patients achieving a CR after R-MVP (rituximab, MTX 3.5 g/m2, vincristine, procarbazine) received dose-reduced WBRT (23.4 Gy), whereas others received standard WBRT (45 Gy). Initial results reported a 2-year OS and PFS of 67% and 57%, respectively, and no treatment-related neurotoxicity was observed.22,23 These results suggest that reduced-dose WBRT achieves disease control comparable to that of full-dose WBRT; neurotoxicity, which was assessed with prospective neuropsychiatric testing, was not observed. A randomized phase 2 study is currently underway comparing R-MPV followed by reduced-dose RT (23.4 Gy), with chemotherapy only (RTOG trial 1114, NCT01399372).
Although microscopic CSF dissemination is common, craniospinal irradiation does not confer additional survival benefit and is associated with significant morbidity.24 No data presently exist to support a role for stereotactic radiosurgery (SRS) for PCNSL. Given the highly infiltrative nature of PCNSL, focal treatments such as SRS would be expected to treat only a small volume of tumor. As a result, SRS is not recommended as a routine treatment for PCNSL.
Chemotherapy
Primary central nervous system lymphoma is a typical NHL histologically, so there have been efforts to treat this tumor in the same manner as its systemic counterpart using cyclophosphamide, hydroxydaunomycin (doxorubicin), Oncovin (vincristine), and prednisone (CHOP). CHOP, or comparable regimens in combination with WBRT, did not improve survival over WBRT alone in PCNSL, and these regimens have been abandoned.
High-dose Methotrexate
High-dose MTX constitutes the backbone of chemotherapy for PCNSL (Fig. 37.2), with efficacy established in prospective phase 2 trials. Unfortunately, the great variability in patient characteristics and regimens used in these studies makes comparisons difficult. MTX doses from 1 to 8 g/m2 intravenously every 10 to 21 days are used in PCNSL (Table 37.3). Total dose and infusion rate are important to achieve concentrations of MTX that cross the blood–brain barrier (BBB) sufficiently. Patients treated with ≥ 3 g/m2 reliably achieve sufficient CSF levels of MTX with a rapid (3-hour) infusion, but even doses as low as 1 g/m2 can achieve therapeutic CSF levels.25 Dose reduction due to impaired creatinine clearance was needed in 45% of patients in trials using MTX 8 g/m2, whereas in the 3.5-g/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree