There is no national cancer care program or system of care in the United States. Efforts to diagnose cancer and coordinate care are left to individual physicians, health plans, and cancer care centers. Health care concerns are magnified with a cancer diagnosis because of the nature of the disease, the complexity of management, the frequent reliance upon new and experimental interventions, and the high costs of care. The continuum of care spans prevention, early detection and screening, diagnosis and treatment of new cases, care of survivors, palliative care, and finally, support for terminally ill patients and their families.
Upon diagnosis of cancer, patients and their families have to cope not only with the diagnosis and an uncertain outcome but also with unfamiliar procedures and the presentation of treatment options. Patients are often asked to make choices between different therapeutic options, and many wish to be involved in the decision-making process of their care. Detailed information on treatment options has a number of beneficial effects, including the ability of the patient to gain control, to reduce anxiety, to improve compliance, to create realistic expectations, to promote self-care and participation, and to generate feelings of safety and security. Increasingly, patients are health consumers and want to be active participants in medical decision-making. As communities have become better educated and information about health care has become more accessible, a fundamental shift in society’s expectations of clinicians has occurred. There is increased accountability of clinicians to standardize medical care according to best medical practices and to improve health care outcome. The quality of information available to patients on health care treatment options has improved, particularly with access to the internet, and with pressure from various consumer advocacy groups, patients now more frequently participate in treatment decisions. For example, the decision making regarding early-stage breast cancer is complex, and the decision-making process is problematic for many patients, especially minority patients.
1 Katz et al.
2 examined the relationship between patient involvement in decision making and type of surgical treatment for women with breast cancer. The authors surveyed a population-based sample of women diagnosed in 2002 with early-stage breast cancer from Detroit and Los Angeles, which are two areas that have previously demonstrated differing practice patterns with respect to breastconserving surgery. Among these women, approximately 70% underwent breast conserving surgery, and 30% received mastectomy. Thirty-seven percent of the women perceived the surgeon to recommend neither surgical procedure over the other. However, when a specific recommendation was perceived by women, breast-conserving surgery was reported as being recommended by the surgeon more often (49% of women) than mastectomy (15% of women). Almost 80% of the women reported making their own decision or sharing the decision with their surgeon. Greater patient involvement in decision-making was associated with greater use of mastectomy rather than greater use of breast-conserving surgery.
There are often many therapeutic options for treating different cancers. Traditionally, most cancers have been treated with surgery, radiation, chemotherapy, or some combination of the three. Surgery is the mainstay of treatment for solid tumors (ie, most cancers except lymphoma or leukemia). For most nonmetastatic cancers, and locally confined tumors, surgery can be curative. Radiation is the primary treatment for some cancers (notably Hodgkin disease and other lymphomas), but is most often used in conjunction with surgery. Chemotherapy (including hormone therapy) may be used alone to treat some cancers (lymphomas or leukemia) but it is used more often in combination with surgery and radiation. In many cases, patients begin a protracted course of chemotherapy after surgery. Surgery and radiation therapy generally attempt to cure localized malignancies, whereas chemotherapy treats disseminated neoplasms. Recently, the advantages of combined therapy have become evident, and an increasing number of patients receive combinations of these three therapeutic approaches.
3 The rationale for such combination therapy comes from observations that surgery is most likely to fail locally at the edges of tumor resection (
positive surgical margins), radiation therapy is most likely to fail in the center of tumors, and chemotherapy is most likely to fail in the presence of bulk disease.
Other types of treatments use the body’s own immune system to resist disease. Biologic response modifiers modeled on the body’s own natural products (eg, interferon, the interleukins, and tumor necrosis factor) are commonly used in conjunction with other treatments. Much of cancer treatment involves managing cancer symptoms such as pain or the effects of treatment. Some effects of treatment are short-lived (eg, nausea or hair loss) but others may be permanent (eg, infertility). Frequently, cancer patients also consider complementary or alternative options to conventional treatment. However, the majority of patients use complementary and alternative medicine to supplement their cancer treatment or help them cope with the treatment and/or its side effects. The more popular therapies appear to be dietary treatments, herbalism, homeopathy, hypnotherapy, and imagery/visualization.
4 A European study indicated that 36% of cancer patients reported using some form of complementary therapy,
4 and most U.S. studies report a higher use, often above 40%.
5,
6 This form of treatment is discussed further in “Complementary
and Alternative Treatment.” The more traditional primary treatments of radiation therapy, chemotherapy and biotherapy, and surgery are discussed further.
RADIATION THERAPY
Radiation therapy is the most widely and frequently used treatment for cancer
7 and is primarily delivered utilizing three different modalities: external beam radiation therapy (EBRT), brachytherapy, or radioimmunotherapy. EBRT delivered via a linear accelerator is the most commonly used therapeutic radiation. Radiation is produced in the form of high-energy x-rays by a device that uses high-frequency electromagnetic waves to accelerate charged particles, such as photons and electrons, through a linear tube. Linear accelerators have the ability to treat with shallow depth penetration (electrons) or deep depth penetration (photons). Research suggests that DNA is the target of the cytotoxic effects of radiation.
8 The unit of radiation is the Gray (Gy), which is equal to 100 rads. EBRT can be administered with high-energy photons or electrons. Dosage is specified by the number of Grays for a number of fractions, eg, 3 Gy for ten fractions. Radiation tolerance is inversely proportional to the daily radiation dose and volume irradiated. Conformal and intensity modulated radiation therapies (IMRTs) are newer external techniques that improve the ability to localize the radiation dose and minimize side effects in adjacent normal tissue. Conformal therapy localizes the radiation dose with multiple fields and can be adapted to targets with irregular contours. IMRT is a refinement of conformal therapy whereby different doses of radiation are delivered to different areas in the same radiation fraction. Intraoperative radiation therapy (IORT) is the delivery of irradiation at the time of surgery and is performed by different techniques, including intraoperative electron beam techniques and high-dose-rate brachytherapy. IORT is usually given in combination with EBRT with or without chemotherapy and surgical resection. The addition of IORT to conventional treatment methods has improved local control as well as survival in many disease sites in both the primary and locally recurrent disease settings.
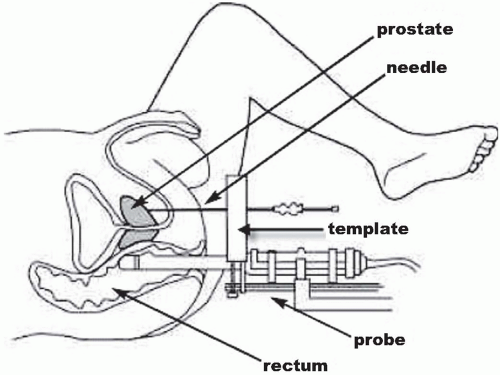
Brachytherapy involves the temporary or permanent placement of selected radioactive sources directly into a body cavity (intracavitary), into tissue (interstitial), into a passageway (intraluminal), or onto a tissue surface (plaque) (
Fig 15.1). Brachytherapy delivers a prescribed treatment dose to a specified tumor volume with a rapid fall-off in radiation dose to adjacent normal tissues. Highor low-dose-rate brachytherapy can be used to treat a number of malignancies, including gynecologic, breast, lung, esophageal, and head and neck cancers, brain and prostate tumors, choroidal melanoma, and others. Brachytherapy can be used as primary treatment or in combination with EBRT to cure or palliate malignancies. By irradiating a small volume of tissue, complications are minimized and organ function can be preserved. Brachytherapy is most often performed using reactorproduced radionuclides such as cesium-137, iridium-192, iodine-125, palladium-103, and gold-198.
With palliative radiation, shorter EBRT schedules that include administering a higher radiation dose with each radiation fraction are generally used. This is called
hypofractionation. It is believed that relief can occur more rapidly due to greater tumor cell kill per fraction. Furthermore, as patient survival is generally shorter, there is less concern about late-onset tissue toxicity. Longer courses with smaller fractions provide more durable pain relief due to larger absolute numbers of tumor cell kill without the increased risk of increased normal tissue toxicity.
Table 15.1 lists cancers commonly treated by conventional radiation.
Radiosurgery is an EBRT technique that uses multiple convergent beams to deliver a high single dose of radiation to a small volume. In radiosurgery, multiple, highly collimated beams of radiation are stereotactically directed toward a radiographically discrete treatment site. The hallmark of all stereotactic radiation techniques is the rapid dose fall-off at the target edges. The most common use is for intracranial lesions, in which a stereotactic frame is applied to the head; high-resolution neurodiagnostic imaging is performed to define the target; imageintegrated three-dimensional (3D) dose planning is performed by high-speed computers; and an accurate and
dependable technology is used to deliver photon energy to the target volume to achieve the desired clinical effect.
Radiosurgery is currently performed with one of two types of high-energy radiation technologies: x-rays, produced by linear accelerators, and the Gamma Knife, producing γ rays. Radiosurgery is used to treat malignant tumors, such as selected cases of brain metastases and malignant gliomas (for which stereotactic radiosurgical boosts are used in conjunction with fractionated radiation therapy), as well as benign tumors (eg, meningiomas, acoustic neuromas, and pituitary adenomas). It has become an important treatment alternative to surgery for a variety of intracranial lesions.
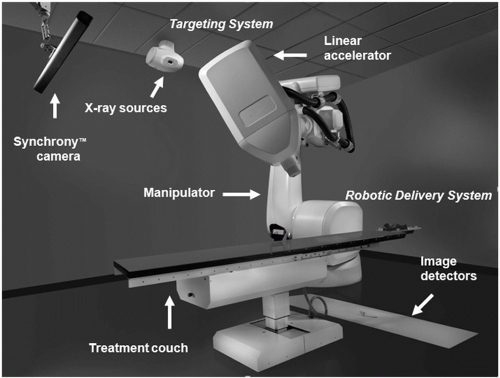
Linear accelerator-based stereotactic radiosurgery techniques have traditionally been used to treat central nervous system CNS tumors. The process combines stereotactic localization techniques, 3D planning imagery, and a sharply focused beam of radiation aimed at a specific, well-defined intracranial lesion. When treating intracranial CNS tumors, patients are positioned in a halo device used for immobilization or noninvasive system with image guidance, primarily to ensure accuracy and reproducibility of the treatment set-up. The CyberKnife is a compact 6-MV linear accelerator (LINAC) that is mounted on a computer-controlled robotic arm and can deliver multiple, nonisocentric, noncoplanar radiation beams. It is essentially a robotic, frameless, image-guided stereotactic radiosurgery system (
Fig 15.2). By using bone landmarks or implanted fiducial markers, stereotactic radiosurgery has been used to treat lesions of the spine, pancreas, prostate, and lung. Because this type of radiosurgery does not require the application of a head frame, staged radiosurgery (ie, fractionation) is feasible. Two standard diagnostic x-ray tubes are rigidly fixed to the CyberKnife treatment room and are set up so that two orthogonal (90-degree offset) images of the target can be obtained. The images are gathered using two amorphous silicon x-ray screens capable of generating highresolution digital images. For initial coarse alignment, identical features from anatomy are visually identified by the operator, and the position of the patient is adjusted by use of a five-axis support table. When the patient’s position is adjusted so that the offset is less than 10 mm, the CyberKnife tracking system automatically compensates for alignment offset and patient motion by adjusting the location of the treatment “isocenter.” In addition, tracking of the position of either a radiopaque (skeletal) target directly or of radiopaque fiducials with known
geometric distances from an x-ray radiolucent target can be performed.
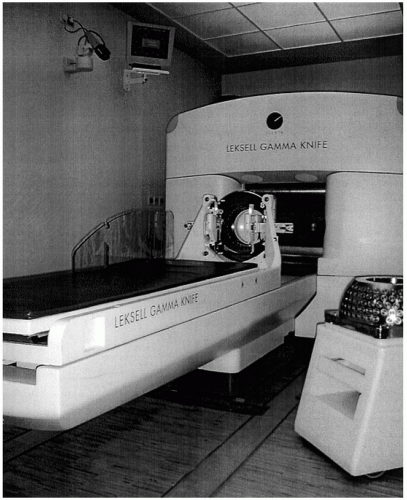
9The Gamma Knife is a self-contained unit with 201 cobalt-60 sources arranged in a hemispheric array such that the emitted beams of radiation reach a common point of intersection (
Fig 15.3). It was designed only to treat intracranial and skull base lesions. The overall time of radiation delivery varies depending on the prescribed dose, but it generally ranges from 15 to 45 minutes. The mechanical accuracy of radiation delivery with use of the Gamma Knife is less than 0.3 mm of variation, due to use of a stereotactic head frame.
High-quality radiation therapy can provide excellent local tumor control for either definitive treatment or palliation. The hallmark of good-quality radiation therapy is adequate tumor coverage while minimizing the risk of injury to normal tissue. This can be achieved by a team of physicians, physicists, nurses, dosimetrists, and therapists who can work as a team to deliver the correct dose to the tumor in the appropriate dose per fraction.
The role of radiation therapy in the management of painful conditions will be discussed further under “Radiation Strategies” in
Chapter 21.
Complications of Radiation Therapy
Damage to normal tissues remains the most important limiting factor in the treatment of cancer by radiation therapy. Patients may experience symptoms associated with damage to normal tissue during the course of therapy for a few weeks after therapy or months or years later (
Tables 15.2 and
15.3). Delayed progression of late effects for 20 to 34 years after therapy has been described.
10 The pathological processes of radiation injury begin immediately after radiation exposure, but the clinical and histologic features may not become apparent for some time. Symptoms may be caused by cell death or wound healing initiated within irradiated tissue, and may be precipitated by exposure to further injury or trauma. Radiation injury is commonly classified as acute, consequential, or late effects, according to the time before appearance of symptoms. Acute (early) effects are those that are observed during the course of treatment or within a few weeks after treatment. Consequential effects (sometimes called consequential late effects) appear later, and are caused by persistent acute damage. Late effects emerge months to years after radiation exposure. Acute radiation damage is most prominent in tissues with rapidly proliferating cells, such as in epithelial surfaces of the skin or gastrointestinal tract. Symptoms develop when functional cells are lost as part of normal tissue turnover and are not replaced because of damage to the stem-cell compartment. Late effects may occur in tissues with a slow turnover of cells, such as subcutaneous tissue, fatty tissue, muscle, brain, kidney, and liver, and in sites of slow turnover within tissues that contain rapidly-proliferating cells, such as the wall of the intestine.
Significant musculoskeletal complications can result from radiation therapy and include muscle fibrosis and atrophy, fractures, and limb length discrepancy. Higher radiation doses (>60 Gy) can result in more pain and larger doses per fraction may lead to more muscle and soft tissue damage. Progression of injury may continue for as long as 10 years.
11 Hormone therapy, chemotherapy, radiation therapy, and castration all directly or indirectly damage bone and lead to loss of bone mass. Bone mineral density is usually measured by dual energy x-ray absorptionometry (DEXA) and this is considered the “gold standard” when performed at the femoral neck or total hip.
12 Bone densimetry results are often reported as t-scores, which represent the difference in the number of standard deviations (SD) between the individual’s bone mineral density (BMD) and the mean value for a group of young adults of the same sex. Normal bone mass is defined by the World Health Organization as BMD within 1 SD of young adult mean (t-score ≥1); osteopenia as increased bone loss, with bone mass between 1 and 2.5 SD below normal (t-score,); and osteoporosis as bone mass >2.5 SD below normal (t-score >2.5).
13 For every SD by which BMD is below peak bone mass, fracture risk approximately doubles.
14Xerostomia is a common side effect encountered by patients receiving radiation to the oral cavity because of the proximity to the salivary glands. It can be transient or permanent, depending on the radiation dose. Evidence shows that mean doses of less than 26 Gy to the parotid glands may avoid permanent xerostomia.
15 Radiation mucositis (radiation-induced mucosal injury) usually occurs 2 to 4 weeks into treatment and abates 3 weeks to 2 months after the completion of radiation. Acute mucositis can be painful, and pain issues need to be addressed immediately to allow the patient to continue to eat. To minimize the chance of secondary infection, most radiation oncologists recommend baking soda mouthwash. If secondary infections (most often thrush) occur, appropriate antibiotic treatment is initiated. Topical anesthetic mouthwashes provide pain relief for 10 to 30 minutes and may help with eating. Many patients require narcotic analgesia for adequate pain control. Mucositis often causes significant swelling, which may be managed by nonsteroidal antiinflammatory drugs (NSAIDs) or steroids. Usually, mucositis resolves 4 to 6 weeks after radiation is completed, but occasionally it can last for several months, necessitating close surveillance. Late complications of EBRT for base of tongue cancers include soft-tissue necrosis/ulceration, osteoradionecrosis (ORN), and xerostomia.
16 The mandible is among the bones most frequently affected by irradiation. ORN of the mandible is a serious late complication of high-dose radiation therapy for tumors of the oropharynx and oral cavity. The diagnosis of ORN is principally based on the clinical picture of chronically exposed bone. Radiological symptoms include decreased bone density with fractures, cortical destruction, and loss of spongiosa trabeculation. Numerous factors that may be associated with the risk of ORN include treatment-related variables (for example, total radiotherapy dose, biologically effective dose, photon energy, brachytherapy dose rate, combination of external beam irradiation and interstitial brachytherapy, field size, fraction size, volume of the mandible irradiated with a high dose), patient-related variables (eg, deep parodontitis, preirradiation bone surgery, poor oral hygiene, alcohol and tobacco abuse, bone inflammation, dental extraction after radiotherapy) and tumor-related factors (tumor size or stage, proximity of the tumor to bone, anatomic tumor site).
17 Primary management of postradiation bone lesions include conservative modalities such as saline irrigations, antibiotics during infectious episodes, topically applied antiseptics, gentle sequestrectomy and removal of visibly loosened bone elements as well as treatment with hyperbaric oxygen. Surgery is reserved for persistent ORN and includes radical resection of the lesion (sequestrectomy, hemimandibulectomy, and so on) with reconstruction.
The risk of injury to the intestine is dose limiting during abdominal and pelvic radiation therapy. Delayed bowel toxicity is difficult to manage and adversely impacts the quality of life of cancer survivors. The rectum is the area most often affected by pelvic radiation for treatment of prostate and cervical cancer. The acute symptoms are diarrhea from loss of integrity of the epithelium and increased secretion of mucus. The most frequent but relatively uncommon late effects include increased stool frequency, urgency, spotting of blood, and partial incontinence. Much less common are ulceration, severe bleeding, pain, stricture, severe incontinence, and fistula. Treatments for rectal complications include: oral anti-inflammatory agents, pain management, stool softeners, intrarectal steroids, transfusions (for bleeding), and dilatation of strictures. For serious or refractory complications, hyperbaric oxygen or surgical intervention with temporary or permanent colostomy may be required. Hyperbaric oxygen therapy significantly improved the healing responses in patients with refractory radiation proctitis.
18Pelvic radiation causes chronic fibrosis and progressive endarteritis in poorly oxygenated bladder submucosal and muscular tissues, with eventual tissue scarring.
19 This can lead to bladder mucosal sloughing and symptomatic hemorrhagic cystitis. Delayed radiation-induced hemorrhagic cystitis may appear more than ten years after pelvic radiotherapy. Traditional treatment methods include bladder irrigation, cauterization, oral or intravenous agents, intravesical chemical instillation, iliac artery embolization, urinary diversion, and cystectomy. However, no single treatment has resulted in satisfactory symptom control in most patients. Hyperbaric oxygen therapy may be a good primary option for the management of hemorrhagic cystitis. 20 One hundred forty-five (76%) of 190 reported patients demonstrated complete or partial symptomatic improvement with hyperbaric oxygen therapy, even among those who had failed multiple previous medical, cystoscopic, or intravesical therapies.
21 Chong et al.
19 reported that delivery of hyperbaric oxygen therapy within 6 months of the onset of hematuria was associated with an increased therapeutic response rate, even in patients with a history of clot retention. In patients with persistent pelvic radiation-induced toxicity (proctitis/cystitis, longstanding vaginal ulcers and fistulas, long-standing skin injuries), hyperbaric oxygen was both safe and effective.
22
CHEMOTHERAPY AND BIOTHERAPY
Chemotherapy consists of drugs that may be given with curative or palliative intent. Adjuvant therapy refers to additional treatment, usually given after surgery.
Adjuvant chemotherapy is given after surgery or radiation therapy in an attempt to prevent tumor recurrence. Its goal is to treat residual micrometastatic disease. Adjuvant chemoradiation is intended to prevent local or regional recurrence. It may be used in patients with positive surgical margins. Adjuvant chemotherapy reduces the rate of recurrence of some tumors, especially ovarian, breast, osteogenic sarcoma, colon cancer, and Wilms tumor. Neoadjuvant therapy, by contrast, is given before surgical resection and/or in addition to radiation therapy specifically for tumor reduction. The most common reason for neoadjuvant therapy is to reduce tumor size before surgical resection.
The majority of chemotherapy is delivered systemically, but regional therapy can also be used. The purpose of regional chemotherapy is to deliver higher concentrations of chemotherapy while minimizing systemic toxicity. Examples of regional administration include intraperitoneal and neuraxial. Chemotherapy is given neuraxially either by lumbar puncture or through an Ommaya reservoir attached to a ventricular catheter. Common intrathecal agents are methotrexate and cytarabine. Hepatic artery delivery of floxuridine (FUDR) via an implanted system in the treatment of colorectal liver metastases represents the largest application of hepatic artery therapy. Most trials have suggested an improvement in both overall and progression-free survival with hepatic artery infusion therapy.
23 Dose-limiting toxicity associated with hepatic artery infusion is related to hepatobiliary sclerosis, which has been reduced with the addition of dexamethasone as part of the treatment.
Chemotherapy for responsive tumors such as lymphoma, small-cell lung cancer, germ cell tumors, and possibly breast cancer may achieve pain relief. Chemotherapy regimens utilizing a combination of agents having different modes of action and exhibiting different forms of toxicity are more likely to cure than single-agent therapy. This is believed to be related to the low probability of double resistance to two drugs which is much less than the risk of single-drug resistance. Since the fraction of cells killed is proportional to the dose employed, maximally tolerated drug doses are indicated. The development of resistant tumor cell clones is related to single drugs, low doses, and long intervals between chemotherapy cycles. High-dose chemotherapy accompanied by autologous hematopoietic stem cell transplants is indicated for the treatment of high-grade non-Hodgkin lymphoma (relapsed) and acute myelocytic leukemia when an allogeneic donor is not available. Some have employed this approach for stage IV breast cancer in remission and to complete the adjunctive therapy of high-risk primary breast cancer.
Emerging evidence has suggested that the capability of a tumor to grow and propagate may be dependent on a small subset of cells within the tumor, termed
cancer stem cells.24 In an animal model, cancer stem cells have the capacity for unlimited self-renewal, as well as the ability to initiate and drive tumor progression. Thus, they would seem the most probable candidates responsible for tumor chemoresistance and recurrence. Before the recognition of cancer stem cells, cancer treatment was traditionally based on the assumption that human cancer cell populations were homogeneous. It was thought that resistance to treatment occurred because malignant cells survived chemotherapy and radiation or avoided immune surveillance of endogenous cytotoxic T cells and natural killer cells.
25 The concept of cancer stem cells may have profound implications for our understanding of tumor biology and for the design of novel treatments targeted toward these cells. Current therapeutic strategies now include targeting the cancer stem cell.
25Patients with metastatic solid tumors have typically been treated with palliative chemotherapy. However, there are situations where metastatic disease is potentially curable. Metastatic testicular cancer, gestational choriocarcinoma, Hodgkin disease, and high-grade lymphomas are potentially curable with chemotherapy.
26 A common feature of such curable tumors is that they arise from cells that undergo major genetic rearrangements or recombination as part of their normal physiology. The absence of further genetic and epigenetic changes in genes that regulate apoptosis, DNA repair, and senescence allows these cells to maintain their intrinsic sensitivity to chemotherapy. This process allows the cells, when challenged with chemotherapy, to undergo the natural apoptotic pathways that contribute to their intrinsic qualities of chemosensitivity and high curability.
Traditional chemotherapeutic agents are cytotoxic drugs that are either cell cycle-specific or cell cyclenonspecific. Antimetabolites such as 5-fluorouracil (5-FU), gemcitabine, and methotrexate are more active on the S phase of the cell cycle. Vinca alkaloids and taxanes work on the M phase of the cell cycle. Cell cycle nonspecific agents such as the anthracyclines (doxorubicin, idarubicin) form free radicals that result in DNA strand breaks. However, these agents are known to have cumulative cardiotoxicity. The camptothecins (irinotecan, topotecan) inhibit topoisomerase I and cause single-strand DNA breaks. The platinums (cisplatin, carboplatin, oxaliplatin) crosslink DNA and inhibit DNA synthesis and transcription. Of note, some agents such as 5-FU can exacerbate symptoms of systemic lupus erythematosus.
27,
28With advances in molecular and cellular biology, antineoplastic therapy has become more refined. Imatinib (Gleevec) is a tyrosine kinase inhibitor that targets an oncogene and platelet derived growth factor. It has been used successfully in the treatment of chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST). Other tyrosine kinase inhibitors include erlotinib (Tarceva) which is used for lung and pancreas cancer, and sunitinib (Sutent) which is used for renal cell carcinoma and GIST. Bortezomib (Velcade), a proteosome inhibitor, is used for multiple myeloma.
Biotherapy utilizes biologicals and biologic response modifiers in the treatment of cancer. Tumors express a wide variety of proteins that can be recognized by the immune system. The immune system of the human organism comprises the innate system cells and the adaptive immune cells. The innate system includes hematopoietic cells, mast cells, basophils, monocytes, dendritic cells, and macrophages. Adaptive cells include CD4
+ T cells, CD8
+ T regulatory cells, and B cells. Biotherapy approaches to cancer treatment aim to protect and reactivate patients’ adaptive immunity against tumor cells.
29 In healthy individuals there is a T helper 1 (Th1) and T helper 2 (Th2) balance, but during microbial-induced inflammation, pathogens induce an overproduction of Th2 cytokines that inhibit adaptive responses against a pathogen.
30 Tumor cells may induce increased Th2 cytokine levels that
can serve as indicators for the existence of tumors.
31 Polarized Th1 cells produce interleukin (IL)-2, IL-12, and interferon-γ. Polarized Th2 cells and hematopoietic cells produce IL-4, IL-5, IL-6, IL-10, and IL-13.
29