Prevention of Waterborne Infections (Including Legionella)
Martha L. Carvour
Loreen Herwaldt
EPIDEMIOLOGICAL CASE STUDY: INTRODUCTION
A 54-year-old hospital visitor was transported from the hospital cafeteria to the emergency department with shortness of breath, cough, and dizziness. The symptoms started <24 hours earlier and progressed rapidly. The patient spent most of the preceding week at her sister’s bedside in the hospital’s intensive care unit, where her sister was recovering from injuries sustained in a motor vehicle accident. The patient slept at a hotel near the hospital each night.
Upon evaluation in the emergency department, the patient was diagnosed with pneumonia. She had a fever, an elevated white blood cell count, and a chest radiograph showing infiltrates in both lungs. She required supplemental oxygen, intravenous fluids, and antibiotic therapy. Her condition gradually improved. Blood and sputum cultures were negative. A Legionella urine antigen test was positive.
Further investigation revealed three other patients with Legionella pneumonia in the hospital. All three were admitted to medical or surgical units on the same floor as the hospital’s intensive care unit and were hospitalized for more than 1 week before the onset of their respiratory symptoms. No other cases of Legionella were identified among hospital visitors or staff.
WATERBORNE INFECTIONS IN HEALTHCARE SETTINGS
Legionella
Is an important cause of both community-acquired and healthcare-associated pneumonia. In 1976, an outbreak of community-acquired pneumonia occurred among attendees of the American Legion Convention in Philadelphia, Pennsylvania,1,2 earning the pneumonia the eponymous title “Legionnaires disease.” Public health scientists identified an aerobic, Gram-negative rod, later named Legionella pneumophilia, as the etiologic agent.1,2 Subsequent investigations revealed that Legionella had caused outbreaks as early as the mid-1960s, including an outbreak of a nonspecific febrile illness known as Pontiac fever in Pontiac, Michigan, in 1968.1 By the 1980s, Legionella-contaminated water supplies and water-containing mechanical equipment in the healthcare environment had been identified as important reservoirs for the organism.3,4
Legionella species are ubiquitous in aquatic and terrestrial environments and in water distribution systems.2,5,6 Today, more than 60 species and 3 subspecies of Legionella have been identified,7 approximately half of which are known to cause disease in humans.2,6 The contribution of different Legionella species to human disease varies geographically.8 In the United States, L pneumophila causes >90% of documented Legionella cases,8 whereas L longbeachae is the most prevalent species in Australia and New Zealand.2,8 L micdadei, L dumoffii, and L bozemanii also cause infections in humans.4,5,9 L pneumophila has multiple serogroups, of which serogroup 1 is the most common cause of hospital outbreaks.10
Legionella Epidemiology and Clinical Impact
According to the U.S. Centers for Disease Control and Prevention (CDC), the number of reported Legionella cases increased 5.5 times between 2000 and 2017.1 Nearly 7500 cases were reported in the United States in 2017,1 but the infection is underdiagnosed and underreported.1,6 Extrapolating from German data,11 Cunha et al. estimated that as few as 5% of cases may be reported to the CDC.6 About 10% of Legionella infections occur in hospital settings,8 and healthcare facilities are among the most frequent sites of Legionella outbreaks.12 Of the 38 Legionella outbreaks investigated by the CDC between 2000 and 2014, 12 (44%) occurred in hotels or resorts, but 5 (19%) occurred in longterm care facilities and 4 (15%) in hospitals.12
The persons at the highest risk for Legionella pneumonia include those aged 50 years or older and those with a history of smoking, recent travel, chronic lung disease, or immunosuppression due to a hematological malignancy, stem cell or solid organ transplantation, or immunosuppressive medication.2,6,8,13 Hospitalized neonates are also at risk.10,13 Clinical manifestations of Legionella infection can range from a mild, febrile illness to a severe pneumonia complicated by multiorgan failure.6 Legionella species are generally susceptible to antimicrobial therapy with macrolides, fluoroquinolones, or doxycycline.
Even with antimicrobial therapy, the estimated case fatality rate for Legionella pneumonia is approximately 10%
overall2; but this may be significantly higher for patients who acquired the infection in healthcare settings, who are immunocompromised, or who are infected with non-serotype 1 Legionella.2 Healthcare-associated cases accounted for 85% of all deaths associated with Legionella outbreaks investigated by the CDC between 2000 and 2014, with a median healthcare-associated case fatality rate of 24% (range 6%-80%).12 Case fatality rates among immunosuppressed patients may exceed 40%.2
overall2; but this may be significantly higher for patients who acquired the infection in healthcare settings, who are immunocompromised, or who are infected with non-serotype 1 Legionella.2 Healthcare-associated cases accounted for 85% of all deaths associated with Legionella outbreaks investigated by the CDC between 2000 and 2014, with a median healthcare-associated case fatality rate of 24% (range 6%-80%).12 Case fatality rates among immunosuppressed patients may exceed 40%.2
Other Waterborne Pathogens
Although Legionella is an important cause of waterborne infections in the healthcare environment, other waterborne organisms also cause infections in healthcare settings (Table 43-1). Waterborne pathogens of significance to healthcare epidemiologists include a broad range of other Gram-negative organisms (eg, Pseudomonas, Sphingomonas, Achromobacter, Stenotrophomonas, Burkholderia, Acinetobacter, Klebsiella, Chryseobacterium), nontuberculous mycobacteria (eg, Mycobacterium smegmatis, M. mucogenicum, M. gordonae, M. chimaera), and molds (eg, Aspergillus, Fusarium).8,13,14,15 In some instances, these organisms may be resistant to multiple antimicrobial agents,8,13,16 making healthcare-acquired infections caused by these organisms especially difficult to treat. Some organisms may produce extended-spectrum beta-lactamases or carbapenemases13,17,18 and may warrant isolation precautions to prevent spread in healthcare settings. Collectively, Legionella and other waterborne pathogens that are present within a built environment or plumbing system are also known as opportunistic pathogens of premise plumbing (OPPP).19
General Principles of Transmission of Waterborne Pathogens
Infections with waterborne pathogens usually result from colonization of surfaces (such as water faucets or medical devices) in the healthcare environment followed by inoculation of the organism into a susceptible patient. Inoculation can occur via aspiration or inhalation (such as by aerosols from water features, cooling towers, or medical equipment), which is the usual route of transmission for Legionella.8 However, Legionella surgical site infections have been caused by cleansing the skin around surgical incisions with tap water,20 and one case of apparent person-to-person Legionella transmission was documented in a community setting.21
Other waterborne infections may be transmitted via direct introduction of the pathogen on a contaminated device (such as a central line or endoscope), on the hands of healthcare personnel (such as when healthcare personnel do not perform proper hand hygiene or when they use contaminated water to perform hand hygiene), or via droplets or aerosols from contaminated plumbing sources (such as sinks or irrigation devices).8,16,17,18 Waterborne infections may occur as sporadic cases or in clusters, depending on the reservoirs in the healthcare environment and the extent and duration of susceptible hosts’ exposure to those reservoirs. Kanamori et al. recently published a thorough review of healthcare-associated, waterborne outbreaks.13
Approaches to Waterborne Infections for Hospital Epidemiologists
Hospital epidemiologists should be equipped to conduct waterborne infection risk assessments at their facilities, oversee the development and implementation of water management programs that reduce these risks, and conduct investigations of suspected outbreaks of waterborne infections when these occur. Thus, epidemiologists should understand the structural and microbiological factors that contribute to the persistence, dissemination, and transmission of waterborne pathogens in the healthcare environment. Epidemiologists should also understand the emerging principles of clinical and environmental testing for waterborne pathogens and the best methods for controlling growth and spread of pathogens within water systems. The sections that follow address each of these factors.
TABLE 43-1 Microorganisms Associated With Waterborne Outbreaks in Healthcare Settings | ||||||||
|---|---|---|---|---|---|---|---|---|
|
PERSISTENCE OF WATERBORNE PATHOGENS IN HEALTHCARE SETTINGS
Structural Factors
Waterborne pathogens can persist in the complex built environments inherent in most healthcare settings. Plumbing systems may become colonized with pathogens at both proximal sites (such as central pipes or cooling towers) and distal sites (such as faucets, fountains, and ice machines).8,22 Sinks used for hand washing or waste disposal are an important potential reservoir of waterborne pathogens.18 Sinks have been linked to numerous outbreaks of antimicrobial-resistant bacteria in the healthcare environment.17,18 Other receptacles or devices, including portable medical equipment (such as bronchoscopes and nebulizers), may be secondarily contaminated by water from colonized plumbing fixtures.22 Examples of water reservoirs in the healthcare environment that may harbor waterborne pathogens are shown in Figure 43-1.2,8,13,22
Hospital epidemiologists should work closely with hospital administrators, engineering and environmental management staff, and clinical personnel to map the flow of water throughout the health system from proximal sources to distal sites, including all sites, receptacles, or devices where patients at risk for waterborne infections might be exposed to tap water from the hospital supply. Water system maps are an important element of epidemiological risk assessments for waterborne infections (see “Infection Control Risk Assessments” below). Staff can use these maps to identify areas in the water supply where microbial growth may be favored and, therefore, where disinfection or decontamination efforts may be most important.
Microbial Factors
Many waterborne pathogens, including Legionella, can persist in water systems within biofilms, which typically render the pathogens more resistant to disinfection and risk mitigation efforts.8 Biofilms form when microorganisms attach to a natural substrate in the environment (such as a storage tank or pipe) and mature into a system of microcolonies encased in a protective, extracellular polymeric matrix generated by the organisms.23 A biofilm may include many different microbial genera and species.23 Bacteria in biofilms are generally more resistant than free-floating (planktonic) bacteria to killing by mechanical and chemical forces.23 Biofilms can become detached from their original substrate—such as during construction affecting existing pipes or other disruptions in water flow—and disseminate to other components of the water system.22,23
Mechanical factors can contribute to biofilm formation in a healthcare water system. Biofilms are most likely to form in areas where water is stagnant, such as “dead legs” (dead-end sections of a plumbing system without regular flow, Fig. 43-1), long horizontal pipes, low-flow devices, and outdated plumbing fixtures.8 Sediment or scale within a plumbing system may also favor biofilm development.22
Legionella may inhabit biofilms in healthcare water systems. Legionella species may be more likely to grow within biofilms generated by other bacterial species than to initiate biofilm formation de novo.23 However, after incorporation into a biofilm, Legionella species may be more resistant to killing than some other organisms.23
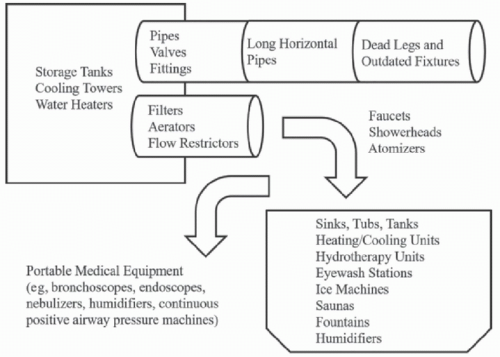 FIGURE 43-1 Potential reservoirs of waterborne pathogens in the healthcare environment.2,8,13,22 Hospital epidemiologists should work closely with hospital administrators, engineering and environmental management staff, and clinical personnel to map the flow of water throughout the health system from proximal sources (such as storage tanks and water heaters) to distal sites (such as faucets, sinks, or medical devices) where patients, visitors, or staff may be exposed. The patterns of flow through the system—and other factors in the plumbing microenvironment (such as temperature, sedimentation, or corrosion)—can contribute to the risk of microbial growth, persistence, and dissemination in the healthcare water supply.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|