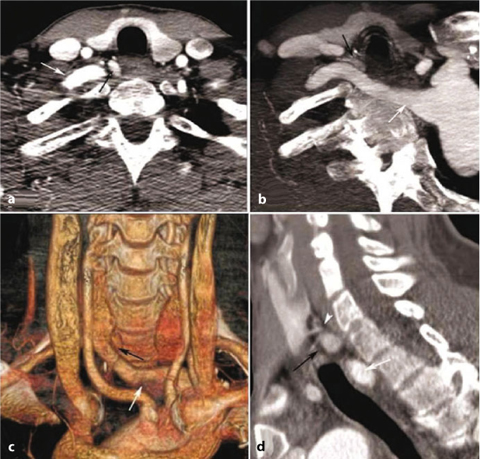
Fig. 9.1
Echo-color-Doppler longitudinal view: left lower hyperplastic parathyroid (thick arrow); left lower thyroid lobe (thin arrow)
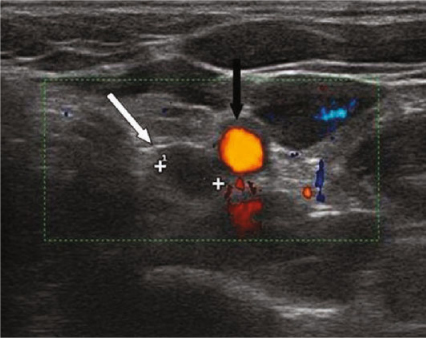
Fig. 9.2
Echo-color-Doppler transversal view: left lower hyperplastic parathyroid (white arrow); common carotid artery (black arrow)
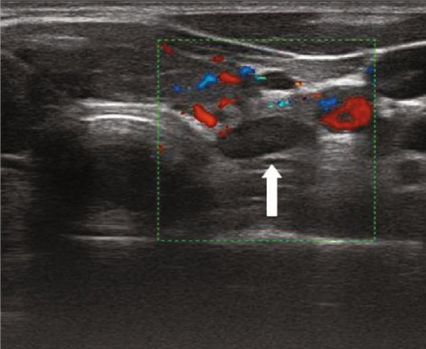
Fig. 9.3
Power-Doppler transversal view: left lower hyperplastic parathyroid (arrow)
Although it is not possible with the use of ultrasound to clearly differentiate between adenomas and hyperplasia, ultrasonography might orientate the differential diagnosis between adenomas and parathyroid carcinomas based on some specific criteria. Data indicate that a malignant lesion should be suspected in the following cases: i) a maximum diameter exceeding 15 mm measured in one of the three axes of the space; ii) a chaotic signal at the power-Doppler either inside or at the periphery of the lesion. The parathyroid carcinoma, in addition, tends to be a round and irregular lesion with a gross thickening of the capsule, a feature suggesting the infiltration of adjacent organs. Moreover, parathyroid carcinoma frequently have a structural heterogeneity and present with a radial vascularization without a clear feeding vessel; these aspects contrast with that of adenomas that have a diffuse vascularization. The cystic degeneration is not helpful for the differential diagnosis since it might be present in both benign and malignant lesions [9] (Fig. 9.4). It is questionable if the sample of the intracystic fluid taken through an ultrasound-guided fine needle aspiration with the measurement of the PTH might be a determinant diagnostic tool in patients in whom the clinical presentation or imaging are not diagnostic.
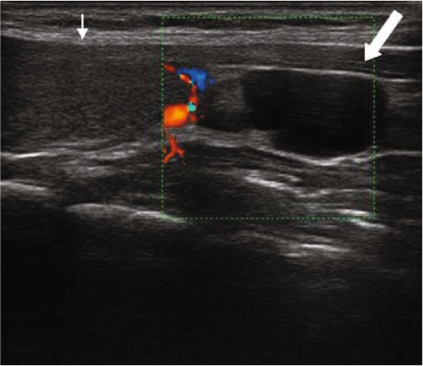

Fig. 9.4
Echo-color-Doppler longitudinal view: cystic degeneration in left lower parathyroid (thick arrow); left lower thyroid lobe (thin arrow)
Second level investigations such as CT and MR have a role in the preoperative planning of ectopic localizations and in the evaluation of regions that cannot be studied by ultrasound. Contrast-enhanced CT identifies parathyroid lesion presenting a contrast enhancement with a diagnostic accuracy of 60%; MR performed with surface coils has the major advantage to identify very small parathyroid lesion down to 5 mm with a diagnostic accuracy of 80%.
9.3 Radionuclide Parathyroid Imaging
The primary objective of parathyroid scintigraphy is to identify and localize the parathyroid glands in patients who have biochemical hyperparathyroidism but are often otherwise asymptomatic. The imaging of parathyroid glands should be highly sensitive, highly specific, as minimally invasive as possible, cost-effective, readily available and safe.
The parathyroid glands, which develop at the sixth gestational week, are usually embedded between the posterior border of the thyroid gland and its fibrous capsule and may be intrathyroidal or mediastinal. Although most patients have four glands, some have five or six [10].
Nowadays, it is commonplace for surgeons to require sensitive and accurate imaging (for preoperative identification and precise anatomical details of any ectopic foci) before performing first surgery or after a failed neck exploration with the aim of a minimally invasive focused procedure [2].
Scintigraphy usually has a lower sensitivity for parathyroid hyperplasia than for parathyroid adenoma. Moreover, in the case of reoperation, imaging is mandatory because it allows for an earlier identification of the offending gland during surgery. The imaging is aimed also at the identification of the ectopic glands and should include single-photon emission CT (SPECT, tomographic acquisition) for a mediastinal focus, or additional CT or MR for confirmation and anatomical landmarks [11,12].
Various scintigraphy protocols may be used: dual-phase single isotope imaging, dual-isotope subtraction imaging or a combination of the two. 99mTc-sestamibi scanning is now considered an optimal imaging technique in patients with hyperparathyroidism. It concentrates in both the parathyroid gland and functioning thyroid tissue, depending on perfusion, cell cycle phase and functional mitochondrial activity.
The scintigraphy, performed with a dual-phase technique, consists of two planar dynamic 10-minute images of the neck obtained by a gamma camera with a pinhole collimator (using a 128 × 128 matrix) after administration of approximately 600–900 MBq of 99mTc-sestamibi; early (10–15 minutes post-injection) and delayed (1.5–2.5 hours post-injection) high count images are obtained (Fig. 9.5). The differential washout is the mainstay of the dual-phase technique: although 99mTc-sestamibi accumulate both in the thyroid and parathyroid mitochondria, its washout phase is delayed in the parathyroid glands compared to normal thyroid tissue (as demonstrated by O’Doerty et al. in 1992)[13].

Fig. 9.5
Dual-phase technique: a early image (thyroid + parathyroid); b delayed image (parathyroid)
To localize abnormal foci in the mediastinum, a delayed planar scintigraphy using a parallel hole collimator is performed 2 hours after the administration of the tracer. All the images acquired are studied and interpreted by the same experienced nuclear medicine physician [12, 14, 15].
A possible second way to image the parathyroid with radioactive tracers is the parathyroid subtraction scintigraphy; the first subtraction technique was introduced by Ferlin et al. in 1983 and is based on the thyroid examination after the i.v. administration of 99mTc-pertechnetate (thyroid) and subsequent digitally subtraction of this image from the one obtained after administration of thallium-201 (thyroid + parathyroid): the remaining image shows only the functioning parathyroid [16] (Fig. 9.6). The mechanism of 201Tl influx in cells is mediated by sodium-potassium ATPase.
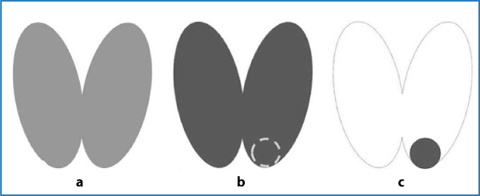

Fig. 9.6
Parathyroid subtraction scintigraphy (201Tl – 99mTc): a 99mTc image; b 201Tl image (thyroid + parathyroid); c 201Tl – 99mTc subtraction image (parathyroid)
However, nowadays, the 99mTc-sestamibi substantially replaced the 201Tl in the parathyroid subtraction scintigraphy given the lower sensitivity and the higher radiation dose of the latter compared to the first.
If no thyroid scan can be obtained, e.g. in the case of poor uptake of 99mTc-pertechnetate in thyroiditis or in case of an assumption of interfering therapy, the method does not provide an adequate result.
The use of US in conjunction with, or after scintigraphy provides increased sensitivity and overall accuracy [17, 18].
Utilization of SPECT/CT hybrid imaging for 99mTc-sestamibi parathyroid scintigraphy improves the test accuracy and may be important to localize ectopic parathyroid adenoma and to plan for a minimally invasive surgical approach for the neck in coexisting nodular thyroid disease [19–21].
On the basis of our retrospective scientific review and experience (based on personal interpretation of about 3000 scintigraphy examinations), we are of the opinion that double phase scintigraphy with 99mTc-sestamibi is readily available, delivers a minimal dose of radiation and has no adverse side effects, offers a superior image quality and has a good cost/efficacy ratio.
When required, preoperative localization should include 99mTc-sestamibi and US scanning rather than assume that the imaging technique is unreliable. Although we agree that the best method of localization is the experienced parathyroid surgeon’s hands [22], an experienced scintigrapher may also provide fundamental information to achieve maximum success [23].
In Figs. 9.7–9.16, some examples of parathyroid scintigraphy are reported.
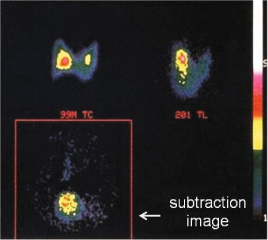
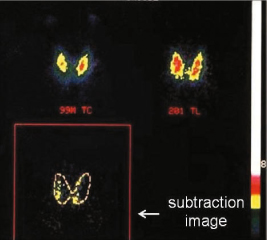
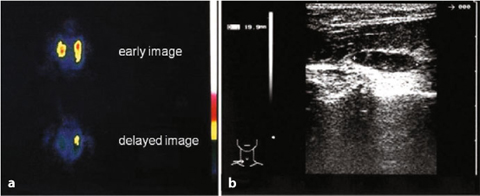
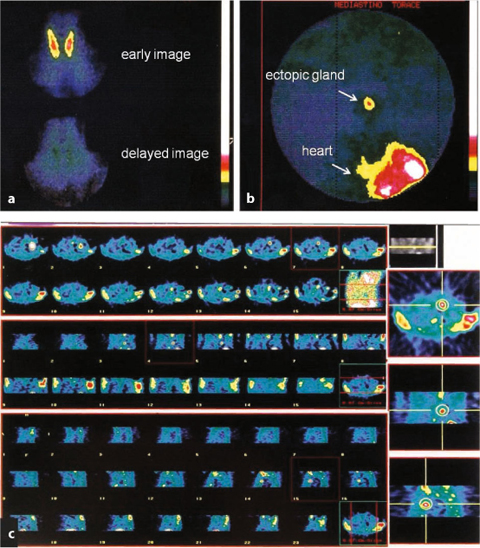
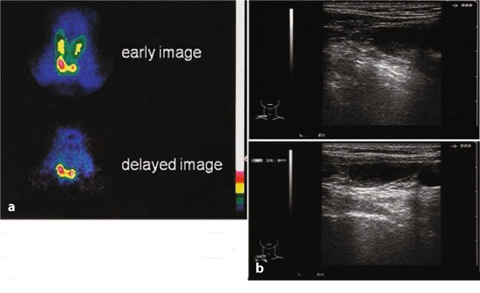
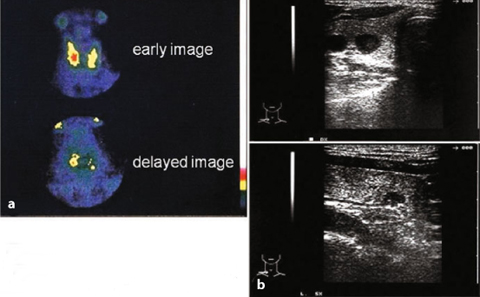
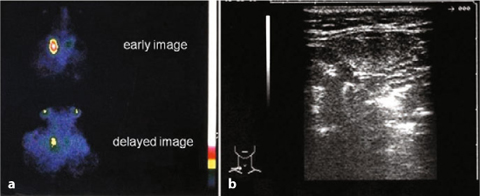
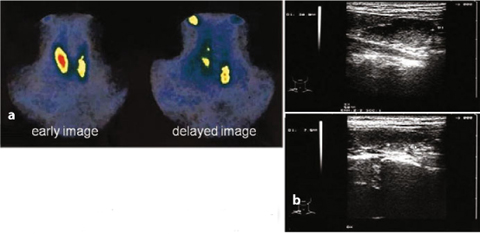
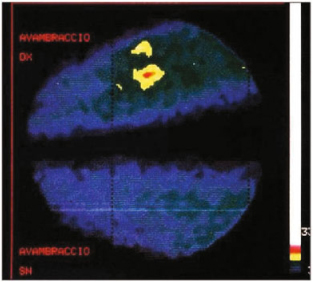


Fig. 9.7
Primary hyperparathyroidism: right solitary adenoma (201T1 — 99mTc)

Fig. 9.8
Primary hyperparathyroidism: the four glands are visible (201T1 — 99mTc)

Fig. 9.9
a Primary hyperparathyroidism (99mTc-sestamibi), adenoma of the left parathyroid gland; b echographic pattern

Fig. 9.10
Primary hyperparathyroidism: a no activity in the neck; b mediastinal adenoma, c SPECT acquisition of mediastinal gland

Fig. 9.11
a Primary hyperparathyroidism (99mTc-sestamibi): b mediastinal gland with negative echography of the neck

Fig. 9.12
a Secondary hyperparathyroidism (99mTc-sestamibi), four glands; b echographic pattern performed on the same day

Fig. 9.13
a Primary hyperparathyroidism (99mTc-sestamibi), post-thyroid surgery; b echographic pattern

Fig. 9.14
a Primary hyperparathyroidism (99mTc-sestamibi), double adenoma; b echographic pattern

Fig. 9.15
Tertiary hyperparathyroidism (201Tl), autotransplant in the right arm

Fig. 9.16
Tertiary hyperparathyroidism (99mTc-sestamibi), autotransplant in the right arm
9.4 Computed Tomography
CT offers an alternative or additional tool in the evaluation of primary hyperparathyroidism. CT is in fact a major tool which provides accurate anatomic information and helps in differentiating adenomas from other mimics, but is often a second-line investigation.
Until the late 1990s, the standard treatment was cervical exploration by a large transverse incision complete with the complications of extensive visceral dissection [24]. In the last decade a minimally invasive approach was adopted and parathyroidectomy needed a more precise spatial imaging. The role of multiple detector computed tomography (MDCT) is thus increased.
To select patients for minimally invasive parathyroidectomy, the surgeon needs precise localization of the parathyroid adenoma in order to exclude multiglandular disease. Indeed mandatory information for the surgeon is number of lesions, site and size of the lesion, and last but not least the probability of being a parathyroid adenoma.
Despite improvements in ultrasound and nuclear medicine technology, neither technique will detect all parathyroid adenomas. CT has advantages common to both US and scintigraphy. It provides excellent anatomic detail for preoperative localization in eutopic and ectopic locations, while the multiple phases show uptake characteristics that help to differentiate parathyroid lesions from lymph nodes and thyroid nodules (Fig. 9.17).