Preexisting Lung Disease and Lung Cancer
Keith M. Kerr
Elsewhere in this book are reviews of the three preinvasive lung diseases that are recognized in the WHO classification of lung cancer as precursors of malignancy. This chapter considers the association between lung cancer and a number of different lung diseases that, apart from causing morbidity and sometimes mortality associated with the underlying pathology of the disease in question, are also associated with a risk of lung cancer development.
By far the most important of these is the association of lung cancer with a variety of forms of diffuse pulmonary fibrosis. Other conditions include a number of cystic conditions that occur in the lung and human papilloma virus (HPV)-induced papillomatosis. It is probable that most of these conditions owe their risk of carcinogenesis to the upregulation of epithelial proliferation that occurs in each case for a variety of reasons. The interaction between this background of cell proliferation and the “usual” lung carcinogens such as those found in tobacco smoke is variable but undoubtedly of importance in some cases.
It is very difficult to say how many lung cancers could be accounted for by transformation of a preexisting lung disease. Many of these conditions are rare. During routine examination of resected lung cancer specimens, the pathologist can sometimes find evidence of what appears to be intercurrent interstitial lung disease. The author has not made a formal study of this but would estimate that such cases account for very approximately 5% of resections. These are, of course, a very select group of lung cancers, due to the patients’ suitability for operation and the resectability of the tumor. The presence of intercurrent disease such as diffuse pulmonary fibrosis is more likely to render the patient unfit for surgery, denying the pathologist the opportunity for full pathological assessment of such cases. On occasion, it may be difficult to distinguish between preexisting lung fibrosis, and that actually caused by the tumor through obstructive pneumonitis. In any such case where the patient smoked tobacco, it is probable that the tumor would be attributed to that particular cause, ignoring the contribution that any concurrent disease may have made.
LUNG CANCER AND PULMONARY FIBROSIS
Localized Pulmonary Fibrosis
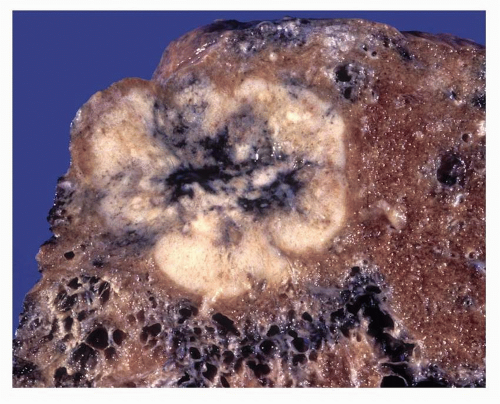
As discussed in Chapter 3, fibrosis is a common occurrence in the center of peripheral pulmonary adenocarcinomas and the development of fibroelastosis, followed by neofibrogenesis are notable features of significance in the development of these tumors. A proposal was made by Rossle in 1943, in which localized scars in the lung could act as a nidus for the growth and development of peripheral forms of lung cancer; the so-called scar cancer or Narbenkrebs hypothesis.1 Beyond the observed presence of central scars in lung adenocarcinomas, anthracotic pigment is often also seen and alveolar cell hyperplasia is recognized in association with lung scars, interpreted as evidence that the scar must predate the growth of the tumor and that fibrosis can stimulate epithelial change, which may lead to malignancy.2,3 and 4 The proposal seemed plausible and was welcome, since the origin of pulmonary adenocarcinoma was otherwise quite unknown. Several reports
followed, documenting cases of peripherally located lung cancer, with central scars.5,6 and 7 Most of these tumors were adenocarcinomas, but squamous and undifferentiated tumors were also described (Fig. 29-1). A majority of these cases were located in the upper lobes. Favored etiology for the scars was tuberculosis and pulmonary infarction. In one large series of almost 1,200 resected lung cancers, 7% of cases were considered scar carcinomas.5 These tumors were not associated with smoking, a finding that probably help acceptance that the scar was the cause of the tumor. A rise in incidence of scar cancer, mostly adenocarcinomas, was described during the 1960-1970s.
followed, documenting cases of peripherally located lung cancer, with central scars.5,6 and 7 Most of these tumors were adenocarcinomas, but squamous and undifferentiated tumors were also described (Fig. 29-1). A majority of these cases were located in the upper lobes. Favored etiology for the scars was tuberculosis and pulmonary infarction. In one large series of almost 1,200 resected lung cancers, 7% of cases were considered scar carcinomas.5 These tumors were not associated with smoking, a finding that probably help acceptance that the scar was the cause of the tumor. A rise in incidence of scar cancer, mostly adenocarcinomas, was described during the 1960-1970s.
The scar cancer hypothesis is now considered incorrect. The above description would fit perfectly well with what we now know about the evolution of adenocarcinomas from atypical adenomatous hyperplasia and localized nonmucinous bronchioloalveolar adenocarcinoma (LNMBAC), effectively adenocarcinoma in situ, and the well-documented rise in the prevalence of adenocarcinoma in many countries. Much of the work in refuting the scar cancer hypothesis was carried out by Shimosato et al.8,9 and 10 (see also Chapter 27), but others contributed to the following observations:
Adenocarcinoma is common in lungs without parenchymal scars.
Examination of patients’ old chest radiographs, taken prior to presentation with cancer, rarely shows a preexisting scar.
Distant metastases from primary lung cancer and metastatic tumors in the lung often show a central scar.
Scars may contain psammoma bodies, suggesting there may have been viable tumor at that site, prior to the onset of fibrosis.
In most cases, the size of the scar and the tumor are in proportion, suggesting they develop in tandem.
Subsequent high-resolution computed tomography studies have shown that the scar indeed develops within the focus of adenocarcinoma in situ (LNMBAC), as a result of alveolar network collapse and fibroelastosis, followed by neofibrogenesis as invasion occurs and stimulates a stromal response.11,12 Growth of the tumors also seems to be accompanied by growth of the “scar”; this is, after all, the invasive part of the lesion. As the scar is the harbinger of invasion, so its appearance is associated with metastatic risk and a poorer prognosis for the patient. Other studies have shown that the central scars in tumors show myofibroblasts and collagen type III, both typical of new fibroblastic growth.13,14 and 15 This contrasts with findings in longstanding localized pulmonary scars that are not associated with cancer. Here, myofibroblasts are few and collagen types I and V are
found. The term “scar cancer” may be retained as a descriptive label, but most evidence suggests the scar is not the cause of the cancer.
found. The term “scar cancer” may be retained as a descriptive label, but most evidence suggests the scar is not the cause of the cancer.
Diffuse Pulmonary Fibrosis
There are many different forms of diffuse pulmonary fibrosis. Among the most important associated with the development of lung cancer are idiopathic pulmonary fibrosis (IPF—Usual interstitial pneumonia), pulmonary fibrosis associated with autoimmune connective tissue diseases (CTDs), and the mineral pneumoconioses, principally asbestosis and silicosis. Why these patients should have a higher risk of developing lung cancer is not entirely clear. Tobacco smoking is probably an importance etiological cofactor. The reactive bronchioloalveolar epithelial hyperplasia, which is common in these conditions, may provide the “at risk” epithelial field within which malignant transformation can take place and asbestos, silica, or other minerals may have carcinogenic properties. Increasing interest has also been shown in the role that inflammation may play in the development of malignancy in the lung.16 In a study of over 1,100 cases of sarcoidosis, however, no excess of lung cancer was found.17
Idiopathic Pulmonary Fibrosis and Lung Cancer
The presence of atypia in the reactive epithelium of IPF and lung honeycombing is commensurate with an association between IPF and malignancy ( Figs. 29-2,29-3,29-4,29-5 and 29-6). There are, however, historical records of the atypical squamous metaplasia occurring in the proliferative and reparative stages of diffuse alveolar damage syndrome being overdiagnosed as squamous cell carcinoma. On the other hand, such squamous epithelium has been shown to bear some genetic changes that are consistent with early progression toward malignancy. Lung cancers have been described in a number of autopsy studies of patients with IPF. In Caucasian cohorts, prevalence of lung cancer reported in IPF autopsies varies from 4.8% to 21% of cases.18,19 and 20 In Japanese studies, the reported prevalence of lung cancer in IPF autopsies appears to be higher at 42% to 48.2%, with 15% of these showing multiple synchronous primary tumors.21,22 and 23 It has been estimated, in a study which controlled for age, gender, and smoking history, that IPF rendered a 14-fold increase in lung cancer risk in males and a sevenfold increase in women.24
Much of the evidence suggests that adenocarcinoma is especially likely to occur in IPF patients. While this is consistent with malignant transformation in the peripheral lung bronchioloalveolar epithelium (see Chapter 27), the data could be confounded by the fact that many studies are of Japanese patients, a population that seems more prone than Caucasians to develop adenocarcinoma.20,23 An excess of adenocarcinoma has, however, been reported in non-Japanese studies.24,25 The finding of an adenocarcinoma predominance in IPF cancers is not universal; some studies have reported a similar distribution of cell types to that seen in a non-IPF smoking populations, over a time period when squamous cell and small cell carcinomas were more prevalent.26,27
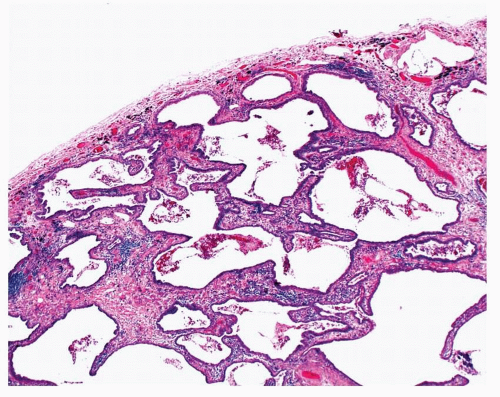 FIGURE 29-2 This patient had a history of IPF. Their lung biopsy showed remodeling of the lung architecture with honeycomb cysts. |
 FIGURE 29-3 In some areas, a transition from nonatypical ciliated bronchiolar epithelium to atypical alveolar lining epithelium is evident. |
 FIGURE 29-4 In other areas, there is atypical epithelium reminiscent of that seen in lepidic-pattern (bronchioloalveolar) adenocarcinoma. |
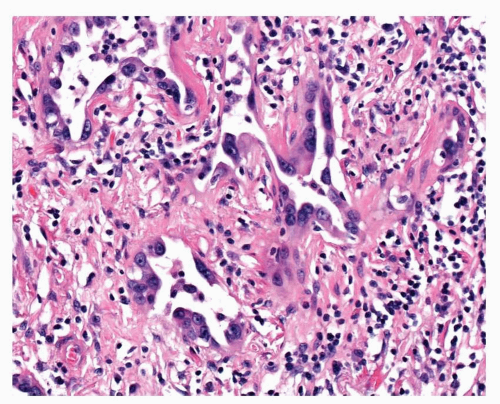 FIGURE 29-5 In some areas, small irregular glandular structures, lined by atypical epithelium, are present. |
 FIGURE 29-6 This patient also had unequivocal invasive poorly differentiated solid-pattern adenocarcinoma. |
IPF-associated cancers tend to occur within or at the margins of the zone of fibrosis and are consequently more often found in the lower lobes. It is possible that the IPF and the malignancy may share common etiology.21,23,27,28 This has been mooted in patients with IPF who have a history of exposure to metal dust20,29 (this does, however, question the diagnosis of IPF, rather than that of pneumoconiosis, but whatever the correct diagnosis, the cases are still relevant to this discussion). IPF is also associated with smoking, raising the possibility of confounding but several studies have controlled for smoking habit and still demonstrated an increased risk of lung cancer in IPF patients.30,31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree