Fig. 18.1
(a) Hypothalamic–pituitary–ovarian axis. (b) Hypothalamic–pituitary–testicular axis
The HPG axis matures during fetal life, with gonadotropin release persisting into infancy. Peaks in gonadotropin and sex steroid levels occur between 6 and 8 weeks of life with levels comparable to those in early to mid puberty. Generally, peripheral effects of these sex steroids do not occur during this “mini puberty,” possibly due to the transient nature of the sex steroid increase. Gonadotropin levels reach a nadir at 6 months of life, although females may retain variable degrees of GnRH release during the first few years of life. The activation of HPG axis early in life is followed by a long period of quiescence through the prepubertal years when hypothalamic activity is suppressed. During the childhood years, the pituitary gland remains responsive to hypothalamic GnRH stimulation, with a characteristic response of greater FSH release than that of LH.
At the onset of puberty, the hypothalamus is reactivated, with enhanced, pulsatile GnRH secretion. Mean levels of FSH and LH increase, with a relatively greater rise in LH. Pubertal onset is initially manifested by LH pulses overnight. LH pulses increase in frequency and amplitude, and extend into the daytime hours as puberty progresses. The exact mechanisms that control hypothalamic GnRH activity and pubertal onset are incompletely understood, but are thought to reflect a balance between inhibitory and stimulatory neurotransmitters of the central nervous system [4–8].
Normal Puberty
Girls
The increase in sex steroids from the gonad, as well as adrenal androgens, is responsible for the physical changes of puberty. In girls, estradiol stimulates the onset and progression of breast maturity, genital growth (elongation of the vulva and growth of the labia minora), maturation of vaginal mucosa, uterine growth, and changes in body composition, mainly accumulation and localization of fat. Breast budding, or thelarche, is often the first sign of puberty in females. However, one-third of girls may experience pubic hair development, pubarche, before thelarche. Progression of breast development and pubic hair growth may be classified into Tanner stages (Fig. 18.2).
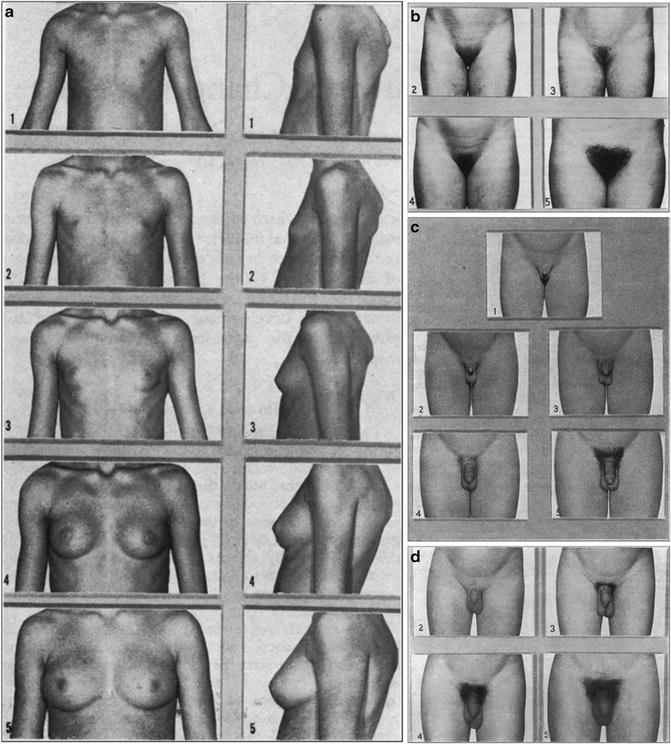

Fig. 18.2
(a) Standards for breast ratings. (b) Standards for pubic hair ratings. (c) Standards for genital ratings. (d) Standards for pubic hair ratings (from Tanner 1969, www.childgrowthfoundation.org)
The adolescent growth spurt is seen early in puberty in girls, driven by the increase in estrogen, and the augmentation of growth hormone release by sex steroids. Menarche occurs approximately 2 years after the onset of puberty. Age at menarche correlates positively with skeletal maturity, and inversely with the remaining height potential. The average girl will gain an additional 4–6 cm after menarche. The duration of puberty is on average 3–4 years, although the tempo of puberty can vary as widely as 2–7 years between individuals.
The historical ranges of normal pubertal timing were based on the data of Tanner and Marshall from the 1960s. In these observational studies of primarily middle-class Caucasian children, 95 % of girls experienced breast development between ages 8.5 and 13 years, with an average age of 11.2 years. Black girls have been found to enter puberty earlier than girls of other ethnic groups, followed by Mexican-American girls, and then white girls [9, 10].
Other data on pubertal timing further support racial differences in the age of onset. These studies show that the early age limit and mean of Tanner 2 breast development for white girls is 8.0 and 10.4 years, for black girls is 6.6 and 9.5 years, and for Mexican-American girls is 6.8 and 9.8 years. The duration between pubertal onset to menarche was found to be approximately 2.3 years in these studies, with median age of menarche of 12.06 years in black girls, 12.25 years in Mexican-American girls, and 12.55 years in white girls [9–16].
Boys
Testicular enlargement is the first sign of normal puberty in boys, with pubic hair growth often occurring around this time. A testicular volume ≥3 ml, or a longitudinal axis of ≥2.2 cm, is indicative of puberty. Axillary hair growth begins at mid-puberty, and hair growth in androgen-sensitive areas progresses. Male puberty can be classified according to Tanner stages of genital development and pubic hair (Fig. 18.2).
Peak growth velocity in boys occurs in mid-puberty with increasing testosterone exposure. Males experience an increase in lean body mass, and a relative decline in body fat during this time. Estradiol levels increase during mid-puberty as the result of conversion of increasing levels of testosterone, potentiating growth and skeletal maturation. Spermarche, which is the onset of sperm production, occurs at an average age of 14 years [17].
The median age of development of Tanner stage 2 pubic hair is 11.2 years in black boys, 12.0 years in white boys, and 12.3 years in Mexican-American boys based on NHANES III data. In the earlier reports from Marshall and Tanner, 95 % of boys entered puberty between 9.5 and 13.5 years with an average of 11.6 years. The average duration of puberty in boys is 3 years [18–20].
Precocious Puberty
Definition
Pubertal development at a younger age than expected for gender and population is considered precocious. The features include progression of pubertal signs, linear growth acceleration, and advancement of skeletal maturity. Classically, pubertal onset before age 8 in girls and age 9 in boys has been defined as precocious. However, racial differences in the timing of pubertal onset must be taken into consideration.
The lower age limit for defining precocious pubertal onset in girls has been controversial. Some experts suggest that the lower age cutoff should be 6 years for black girls and 7 years for white girls, with individuals older than these limits evaluated only if they meet certain criteria: significant skeletal advancement, predicted adult height < 2SD below genetic target, features suspicious of a central nervous system (CNS) lesion, rapid tempo of puberty, or psychosocial concerns. These recommendations have not been universally endorsed due to concerns that the lower age cutoff would fail to identify pathology in some individuals. For example, a retrospective review of patients referred for precocious puberty to a tertiary care center found that application of these lower age limits would have resulted in failure to identify treatable etiologies (such as congenital adrenal hyperplasia, pituitary adenoma, and neurofibromatosis) in 12 % of patients [21].
Until more data are available, careful evaluation of children with signs of secondary sexual development is warranted for girls younger than 8 years and boys less than 9 years. A comprehensive history and physical exam with clinical follow-up may be sufficient in those individuals between the lower and upper suggested age cutoffs whose evaluations indicate slow progression that does not raise concerns for underlying pathology [22].
Normal Variants
When evaluating children referred for precocious puberty, normal variants must be considered. For instance, premature thelarche is isolated breast development in girls without other signs suggestive of true puberty. Premature thelarche often occurs within the first 2 years of life, with a second peak between ages 6 and 8 years. Breast development in these patients is minimally or non-progressive, and is not associated with linear growth acceleration or skeletal age advancement. In most cases, there is no underlying pathology, although some instances have been associated with the use of cosmetic or hair products containing lavender oil, tea tree oil, or placental extracts. Clinical follow-up is warranted in these individuals to monitor for pubertal progression as some may progress to true central precocious puberty [23–26].
Premature Adrenarche
Adrenarche is the component of puberty that is not dependent on the HPG axis and may, therefore, vary in time of onset in relation to HPG activation. Adrenarche results when the adrenal cortex matures and increases production of androgens, including dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEA-S), and androstenedione. Androgens are responsible for some of the physical changes of puberty such as acne, growth of sexual hair, and body odor from the development of apocrine glands. The appearance of these physical signs is referred to as pubarche. Adrenarche usually occurs just before or around the onset of puberty.
Adrenarche before the age of 8 years in females and 9 years in males is referred to as premature. The factors initiating premature adrenarche are not clearly understood. Children with benign premature adrenarche have features that are non- or slowly progressive. In addition, these children do not have growth acceleration, substantial bone age advancement, or other signs of true puberty such as breast development or testicular enlargement. Levels of DHEA, DHEA-S, and androstenedione are often moderately elevated and consistent with Tanner stage of pubic hair. A small percentage of individuals with premature adrenarche may have a pathological cause such as central precocious puberty, nonclassical congenital adrenal hyperplasia, or androgen-producing tumor. Therefore, thorough clinical evaluation and follow-up are warranted in this group of patients. In addition, premature adrenarche has been associated with insulin resistance and polycystic ovarian syndrome later in life [27–31].
Differential Diagnosis
Precocious puberty may occur either by premature activation of the HPG axis which is termed central, or GnRH-dependent precocious puberty (GDPP), or by sex steroid exposure that is independent of hypothalamic–pituitary control, termed peripheral, or GnRH-independent precocious puberty (GIPP). The differential diagnosis is outlined in Table 18.1.
Table 18.1
Differential diagnosis of precocious puberty
Category | Presentation | Diagnosis | Treatment |
|---|---|---|---|
GnRH dependent (central) | |||
Idiopathic | Pubertal changes in normal sequence, although may be more rapidly progressive Female:male >10:1 | Pubertal gonadotropins and sex steroids , either basal or stimulated Skeletal age advanced | GnRH agonist |
CNS abnormalities Tumors Hypothalamic hamartomas Craniopharyngeomas Optic gliomas (may be associated with neurofibromatosis) Structural abnormalities Septo-optic dysplasia Hydrocephalus Arachnoid cysts Intracranial abscesses Other causes Granulomatous disease Infiltrative disease Trauma Surgery Radiation | Features of pubertal development similar to idiopathic Clinical symptoms or signs of underlying CNS abnormality | Similar to idiopathic Imaging (MRI or CT) showing CNS lesion in cases of tumor or structural abnormality | Therapy aimed at underlying lesion GnRH agonist in cases of hypothalamic hamartoma and some CNS lesions |
Chronic exposure to sex steroids with advanced maturation | Initial development out of sequence and/or progresses rapidly due to peripheral cause HPG activation results in onset of true puberty with features similar to idiopathic | Similar to idiopathic Laboratory findings consistent with underlying peripheral etiology (e.g., CAH) | Treatment of underlying disorder GnRH agonist required in most cases |
GnRH independent (peripheral) | |||
General features | Isosexual or contrasexual pubertal changes which may progress rapidly and/or out of normal sequence | ||
Adrenal tumors Adenoma Carcinoma | Virilization in girls, and precocious sexual development in boys Gonads prepubertal | Elevated adrenal androgens such as DHEA, DHEA-S, androstenedione Other adrenocortical hormones such as cortisol may be elevated Imaging revealing adrenal tumor | Therapy directed at tumor including surgery and possibly chemotherapy |
Ovarian tumors Carcinoma Gonadoblastoma Granulosa cell Theca cell | Rapid pubertal progression in girls; feminization in boys | Elevated estradiol Prepubertal gonadotropins CT or ultrasound showing ovarian tumor | Tumor resection |
Testicular tumors Leydig cell | Virilization in girls, and precocious sexual development in boys Asymmetrical testes in boys | Elevated testosterone Prepubertal gonadotropins CT or ultrasound showing testicular tumor | Tumor resection |
Gonadotropin or hCG producing Choriocarcinoma Dysgerminoma Hepatoblastoma Teratoma | Pubertal development with testicular enlargement in males Rare in females; associated with isosexual pubertal development | Elevated LH, FSH, or β-hCG Elevated sex steroids Evidence of tumor on imaging | Tumor resection Chemotherapy and radiation in some cases |
Congenital adrenal hyperplasia | Premature pubarche and rapid growth Virilization in females Gonads prepubertal May lead to central precocious puberty | Elevated 17-OH progesterone and other adrenal precursors either basally or in response to ACTH stimulation | Glucocorticoid and mineralocorticoid replacement |
Male-limited familial precocious puberty (testotoxicosis) | Occurs in males Virilization and rapid growth early in life Testicular enlargement | Pubertal testosterone levels Prepubertal gonadotropins | Ketoconazole Aromatase inhibitors Antiandrogens |
McCune–Albright Syndrome | Isosexual development that occurs in unusual sequence Affects girls>boys Polyostotic fibrous dysplasia Café au lait lesions Other endocrinopathies | Pubertal sex steroid levels Pelvic ultrasound in females may show ovarian cysts GNAS1 mutation in samples from affected tissue | Tamoxifen Aromatase inhibitors GnRHa if central precocious puberty develops |
Primary hypothyroidism | Isosexual pubertal changes associated with lack of expected growth acceleration Gonadal enlargement | Elevated TSH and low thyroxine level Gonadotropins prepubertal | Thyroid hormone replacement |
Exogenous exposure to sex steroids | Various presentations depending upon substance | Variable; some compounds measurable in serum Gonadotropins prepubertal | Identify and remove exposure to compound |
Gonadotropin-Dependent Precocious Puberty
In GDPP, there is premature maturation of the HPG axis, with pubertal changes resulting from centrally mediated sex steroid production. The cause of the early onset of HPG activation may be congenital or acquired CNS abnormalities (e.g., hypothalamic hamartoma, astrocytoma, granulomatous disease, trauma). GDPP may also be idiopathic, which is estimated to occur 10–20 times more commonly in females compared to males. In addition, chronic exposure to sex steroids such as with untreated congenital adrenal hyperplasia may accelerate hypothalamic maturation and HPG activation resulting in GDPP.
In GDPP, the timing and sequence of pubertal events evolve in a normal pattern. The clinical signs are those of normal puberty including development of secondary sexual characteristics, linear growth acceleration, skeletal age advancement, and pubertal levels of sex steroids and gonadotropins.
Gonadotropin-Independent Precocious Puberty
In GIPP, pubertal changes are induced by sex steroids from exogenous sources or from endogenous production that is not directed by hypothalamic GnRH secretion. Endogenous production of steroids may be derived from either gonadal or non-gonadal tissue. For example, precocious puberty may develop in individuals with elevated levels of adrenal androgens such as with congenital adrenal hyperplasia or adrenal tumors. Ovarian and testicular tumors secreting sex steroids may cause early pubertal development. In addition, tumors such as choriocarcinomas or hepatoblastomas can produce gonadotropins and cause GIPP. Gonadotropin receptors may be activated independently of gonadotropin activity, as in LH receptor-activating mutations. For instance, McCune–Albright syndrome (MAS) involves constitutive activation of G-protein-coupled receptors signaling through Gs protein; the receptors for LH, FSH, TSH, GHRH, PTH, ACTH, and MSH function by this mechanism, and activation of these receptors can result in a variable number of endocrinopathies including precocious pubertal development. A more specific form of GIPP is seen in boys with dominantly transmitted sex-limited activation of the LH receptor at a young age. Rarely, long-standing untreated primary hypothyroidism may result in GIPP, thought to be due to chronically elevated TSH levels stimulating structurally similar LH receptors, known as the Van Wyk–Grumbach syndrome [32–35].
Key Points to the Diagnosis
Which Children with Early Pubertal Development Should Be Evaluated?
In general, females less than 8 years and males less than 9 years with pubertal signs should be evaluated. Younger age heightens the concern for a pathological etiology, and warrants a more extensive evaluation. In those children who are approaching the lower age limit, a careful history, physical examination, and bone age determination may be sufficient if no other concerns arise during the initial evaluation.
Are the Pubertal Changes Progressive? If so, What Is the Sequence and Tempo of Changes?
Precocious puberty is associated with progressive pubertal changes which can be differentiated from normal variants such as benign premature thelarche in which pubertal signs are non- or minimally progressive. Precocious puberty is associated with advancement of skeletal maturity and linear growth acceleration, features which are not seen in normal variants of development.
In GDPP, pubertal changes occur in a normal sequence with a tempo similar to normal puberty. On the other hand, children who have GIPP are more likely to have features of puberty outside of the normal sequence, and with aberrant timing. For example, a young boy with development of sexual hair and virilizing features without testicular enlargement is likely to have a peripheral source of androgen production such as an adrenal tumor. Furthermore, children who have a normal sequence of pubertal changes, but have rapid tempo of changes, are more likely to have GIPP. For instance, a young girl with breast development who progresses to menarche within 12 months is likely to have a peripheral source of estrogen production such as an ovarian tumor.
Are the Pubertal Signs Suggestive of the Source of Sex Steroids?
Elevated estradiol levels will result in signs of puberty in girls, but will lead to feminization in males, with gynecomastia. Conversely, elevated androgen levels in females will lead to signs of virilization including hirsutism, acne, and clitoromegaly, but signs of puberty in males. Therefore, isolated virilization in females suggests a peripheral etiology, and excludes GDPP. Feminization in males also excludes a central etiology, and most testicular etiologies with the exception of rare testicular tumors such as a feminizing Sertoli cell tumor.
Does the Patient Have Features of an Underlying Disorder?
Neurological signs such as changes in gait or vision, or symptoms of pituitary hormone deficiencies such as diabetes insipidus or growth hormone deficiency, raise suspicion for CNS abnormalities.
MAS should be considered if there are café au lait spots, bone deformities on the face or long bones, or accelerated growth. In addition, patients with MAS often develop pubertal signs that are outside of the normal sequence. For example, menstrual bleeding often precedes breast development in girls with this disorder, and may occur as early as 4–6 months of age.
Neurofibromatosis type 1 (NF1) may be associated with GDPP, usually but not exclusively in those with optic pathway gliomas. Primary features of NF1 are café au lait macules, axillary freckling, neurofibromas, optic nerve gliomas, and hamartomas of the iris (Lisch nodules).
Signs of precocious puberty with absent linear growth are suggestive of primary hypothyroidism, although this is extremely rare with only approximately 12 reported cases in the literature [36].
In patients with primary adrenal failure with precocious puberty, a DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) mutation should be considered. Rarely, patients with adrenal hypoplasia congenital (AHC) due to DAX-1 mutation may develop GDPP; these individuals may have primary adrenal failure during infancy. DAX-1 mutations may also be associated with Duchenne muscular dystrophy and glycerol kinase deficiency due to a contiguous gene deletion syndrome.
Evaluation
Medical History
Pertinent history includes the age of onset of pubertal signs and their rate of progression, as well as timing of puberty in family members. Growth data are helpful to determine if there is growth acceleration. Symptoms of a CNS lesion such as headaches or seizures should be sought. Exposure to exogenous hormones should be explored; this may be from the cosmetic products noted above or contact with a parent’s topical testosterone [37, 38].
Physical Examination
Important anthropometric data include height, weight, and height velocity (if possible). A thorough neurological examination including visual fields and fundoscopic examination should be performed to evaluate for a CNS lesion. Careful examination of the skin may provide clues to the diagnosis; for example, café au lait macules suggest MAS or NF1.
Pubertal signs should be assessed to determine Tanner stage (previous figures). In girls, the diameter of glandular breast tissue and areola should be measured. Examination of the genitalia for signs of maturation may be helpful. In addition to the labial and vulvar changes noted above, an increase in clear mucous secretions and changes in vaginal mucosa from a thin, red mucosa to a thickened, pink-appearing mucosa are seen in girls with puberty.
In boys, testicular size and pubertal staging should be determined. Testicular volume ≥3 cc or longitudinal axis ≥2.2 cm suggests gonadotropin stimulation and likely GDPP. In a male with pubertal signs, but without testicular enlargement, a peripheral source of sex steroids should be sought. For instance, boys with constitutive activation of the LH receptor in the testis have relatively small testes for their degree of sexual maturation. If significant asymmetry of the testes is present, a testicular tumor such as a Leydig-cell tumor should be considered; some asymmetry, however, is common.
Imaging Studies
A bone age film to determine skeletal maturity should be obtained in children with early pubertal development, recognizing the subjectivity and normal variation of this test. Significant skeletal advancement is present in GDPP, along with other signs such as growth acceleration and pubertal findings on physical examination. In individuals with signs of puberty which are progressive without bone age advancement, a peripheral source should be sought; more rapid increases in sex steroid levels can occur with GIPP compared to GDPP, and ossification of the growth centers lags behind sex hormone stimulation by some 6 months. In addition, those with normal variants of puberty such as premature adrenarche have skeletal age that is normal or slightly advanced, and pubertal signs that are nonprogressive.
Pelvic ultrasonography in girls to assess ovarian and uterine volumes can be useful in establishing whether an individual is pubertal, and for monitoring the progress of puberty. In cases of GIPP, abdominal-pelvic ultrasound should be performed to evaluate for an ovarian tumor or cyst. In addition, a testicular ultrasound study may be indicated in boys with GIPP to evaluate for a testicular mass [39].
Imaging of the CNS with magnetic resonance imaging (MRI) is indicated in most children with GDPP to evaluate for an intracranial lesion. There has been conflicting data about whether MRI should be performed in low-risk categories such as girls over the age of 6 years with GDPP. For example, one series reported no CNS abnormalities found in a group of females with the onset of GDPP after 6 years of age with estradiol levels <12 pg/ml. However, another report found that 15 % of girls in this age group had CNS lesions. A careful neurological examination, consideration of ethnicity, and estradiol levels can be used to determine whether an MRI of the brain is indicated in girls older than 6 years with GDPP. The vast majority of the brain lesions found in these girls are nonprogressive hamartomas that do not require surgical intervention. As idiopathic GDPP is less common in boys, an MRI of the brain is warranted in all males due to the higher likelihood of identifying intracranial pathology [40–42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree