Practical Issues Related to Ancillary Testing of Colorectal Carcinoma
Wade S. Samowitz
Recent advances in our understanding of colorectal cancer progression have led to discovery of important molecular alterations that are biologically relevant for several reasons. Some changes, such as mutations affecting tumor suppressor genes and DNA mismatch repair genes, underlie heritable cancer syndromes, and thus, evaluation for their presence helps identify patients and their family members with heritable cancer risk. Other molecular abnormalities have therapeutic implications. An increasing number of oncogenic mutations affecting membrane-bound receptors can be exploited, or manipulated, to develop novel interventions, whereas other changes, such as KRAS mutations, abrogate the effects of some chemotherapeutic agents. Tumor evaluation for the presence of these alterations is essential to cancer management, and thus, clinicians increasingly demand that ancillary testing be performed on pathology materials. Unfortunately, many clinicians are not fully aware of the limitations of molecular studies, or they inappropriately utilize resources to obtain information that may not be clinically relevant. As a result, pathologists play increasingly important roles as physician scientists and educators regarding the judicious use of molecular assays. The purpose of this chapter is to discuss practical aspects of ancillary testing with focus on caveats unique to the evaluation for mismatch repair deficiency.
EVALUATION FOR DNA MISMATCH REPAIR DEFICIENCY
Overview
The Amsterdam criteria are historical guidelines originally set forth to select individuals for germline testing of DNA mismatch repair genes. These criteria primarily identify patients with cancer onset at a young age or a strong family history, but may fail to detect cancer risk in patients who lack a detailed family history. The revised Bethesda criteria not only include recommendations based on family history and age of cancer onset, but they also take into account important histologic features characteristic of microsatellite unstable tumors.1 Unfortunately, some patients with Lynch syndrome still escape detection by these revised criteria, and thus, many centers now perform universal microsatellite instability (MSI) testing on all colorectal cancers.2, 3 and 4 Mismatch repair deficiency is evaluated using two techniques: PCR amplification of a panel of microsatellite repeats and immunohistochemistry for DNA mismatch repair proteins. The Bethesda consensus panel for microsatellite analysis consists of two mononucleotide and three dinucleotide repeats and is widely used to assess for MSI. However, instability in mononucleotide repeats is probably more important than instability in dinucleotide repeats for detection of Lynch syndrome-related tumors.5,6 Thus, many centers now use a commercially available panel of five mononucleotide repeats, as discussed in Chapter 11.
Interpretation of Immunohistochemistry for Mismatch Repair Proteins
Immunohistochemistry for mismatch repair proteins, MLH1, MSH2, MSH6, and PMS2, is a fairly good surrogate test for microsatellite status. As with all immunohistochemistry stains, however, immunostain results may be difficult to interpret in some situations. Immunohistochemical
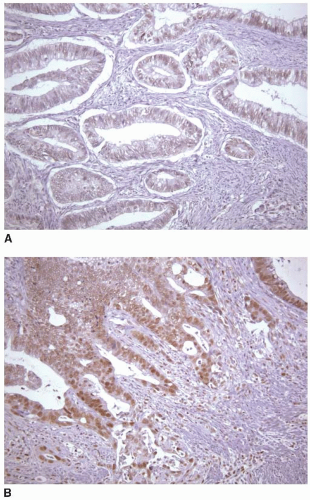
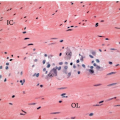
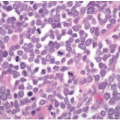
staining quality is dependent on a variety of factors, including type of fixative, time interval between removal of the specimen from the patient and fixation, adequacy of tissue fixation, and duration of fixation. The effects of these variables on immunohistochemical staining of colorectal carcinomas have not been studied as systematically as they have been in breast cancer, for example, but they probably affect extent and intensity of staining, which can vary between different areas of a single slide. Fortunately, normal staining of nonneoplastic cells, such as lymphocytes, stromal cells, and colonic crypts, serves as an internal control for DNA mismatch repair protein immunohistochemistry. Only areas of the slide with intact internal control staining are used to evaluate tumor staining, and, in fact, areas that show a lack of internal control staining should be classified as uninterpretable (Figure 12.1). Tumor-infiltrating lymphocytes are a common histologic feature of microsatellite unstable colon cancers and represent a very useful internal control for immunohistochemistry interpretation (Figure 12.2).7 Mismatch repair protein expression tends to parallel proliferative activity, so cancer cells, germinal centers, and stromal cells in granulation tissue generally show strong nuclear staining. Cells that are not actively proliferating, including surface epithelial cells in the normal colon, show a lack of, or diminished, staining compared to proliferating cells. Thus, it is not uncommon to see negative, or faintly positive, nonproliferative cells, such as lymphoid cells surrounding highly proliferative germinal centers and superficial epithelium of serrated polyps. For this reason, a lack of immunostaining in some, but not all, tumor cells does not necessarily imply the presence of DNA mismatch repair protein deficiency, even if there is intact staining of internal controls.
staining quality is dependent on a variety of factors, including type of fixative, time interval between removal of the specimen from the patient and fixation, adequacy of tissue fixation, and duration of fixation. The effects of these variables on immunohistochemical staining of colorectal carcinomas have not been studied as systematically as they have been in breast cancer, for example, but they probably affect extent and intensity of staining, which can vary between different areas of a single slide. Fortunately, normal staining of nonneoplastic cells, such as lymphocytes, stromal cells, and colonic crypts, serves as an internal control for DNA mismatch repair protein immunohistochemistry. Only areas of the slide with intact internal control staining are used to evaluate tumor staining, and, in fact, areas that show a lack of internal control staining should be classified as uninterpretable (Figure 12.1). Tumor-infiltrating lymphocytes are a common histologic feature of microsatellite unstable colon cancers and represent a very useful internal control for immunohistochemistry interpretation (Figure 12.2).7 Mismatch repair protein expression tends to parallel proliferative activity, so cancer cells, germinal centers, and stromal cells in granulation tissue generally show strong nuclear staining. Cells that are not actively proliferating, including surface epithelial cells in the normal colon, show a lack of, or diminished, staining compared to proliferating cells. Thus, it is not uncommon to see negative, or faintly positive, nonproliferative cells, such as lymphoid cells surrounding highly proliferative germinal centers and superficial epithelium of serrated polyps. For this reason, a lack of immunostaining in some, but not all, tumor cells does not necessarily imply the presence of DNA mismatch repair protein deficiency, even if there is intact staining of internal controls.
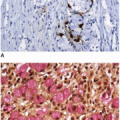
Finally, it is generally accepted that colon cancers with PCR-proven MSI show complete loss of immunohistochemical staining for one or more antibodies to DNA mismatch repair proteins depending on the nature of the underlying deficiency. This is especially true for sporadic unstable tumors due to acquired MLH1 methylation because epigenetic silencing of transcription turns off mRNA expression and completely abrogates protein expression. However, we now recognize that some colon cancers due to Lynch syndrome show markedly decreased DNA mismatch repair protein immunoexpression, but the staining reaction is not completely lost.8 Although overemphasizing the importance of diminished staining intensity may result in “false positives” and unnecessary germline testing, such factors do warrant consideration. We typically recommend evaluation by PCR to help evaluate the significance of markedly decreased staining intensity, especially if the staining reaction is diminished relative to that of the internal control.
Heterodimers formed by DNA mismatch repair proteins (MLH1/PMS2 and MSH2/MSH6) affect interpretation of immunostain results, as discussed in Chapter 11 (Table 11.1). Stability of PMS2 and MSH6 is highly dependent on these complexes, whereas stability of MLH1 and MSH2 is not. Therefore, combined loss of MLH1 and PMS2 immunostaining reflects defective MLH1, and combined loss of MSH2 and MSH6 suggests an abnormality in MSH2. Isolated loss of MSH6 or PMS2 immunostaining usually indicates a defect in MSH6 or PMS2, respectively.9 However, there are exceptions to these rules. Isolated loss of PMS2 immunostaining can reflect an MLH1 germline mutation when the mutation results in nonfunctional, but antigenic, MLH1 protein.10 “Clonal” loss of MSH6 is occasionally encountered in some, but not all, areas of a tumor.9 This usually occurs in cancers with MSI due to other deficiencies, such as MLH1 promoter methylation. Underlying MSI in these cancers probably causes instability in coding mononucleotide repeats of MSH6 through mismatch repair deficiency, leading to inactivation of MSH6 protein.11
Distinction between Sporadic and Hereditary Colorectal Cancers with Microsatellite Instability
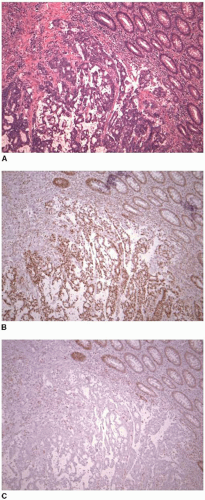
The results of DNA mismatch repair protein immunohistochemistry facilitate subsequent evaluation for possible Lynch syndrome (Figure 12.3). Combined loss of MSH2 and MSH6 and isolated loss of MSH6 or PMS2 are most likely indicative of Lynch syndrome.12 However, the molecular basis for most sporadic colon cancers with MSI is MLH1 promoter methylation, which results in loss of immunostaining for both MLH1 and PMS2. This pattern of staining is also expected in heritable tumors due to a germline defect in MLH1. Sporadic colon cancers with MSI are far more common than those associated with Lynch syndrome, and thus, differentiating sporadic from MLH1-deficient Lynch syndrome???related tumors before proceeding to germline testing avoids unnecessary expense for the majority of microsatellite unstable cancers. Fortunately, other molecular features of cancers with MSI can be evaluated to facilitate this distinction. Approximately 50% of sporadic colon cancers with MSI harbor a mutation in BRAF, a serine???threonine kinase.13 The mutation is typically a “hot spot” valine to glutamate substitution at codon 600 (V600E) and has only rarely been reported in unstable tumors associated with Lynch syndrome.14 Therefore, colon cancers with MSI that harbor the V600E BRAF mutation are very likely to be sporadic in nature, and germline evaluation for Lynch syndrome is probably unnecessary. In addition, assessment for MLH1 promoter methylation can also be used to distinguish between sporadic and hereditary microsatellite unstable tumors.15 Sporadic colon cancers with MSI develop as a result of MLH1 promoter methylation, which has only rarely been reported in Lynch syndrome???associated tumors. Rare cancers with a Lynch syndrome phenotype due to germline methylation of the MLH1 promoter are distinguished from sporadic tumors by the presence of MLH1 promoter methylation in nonneoplastic tissue.16
Two important points must be considered when using BRAF mutational status and MLH1 methylation to distinguish sporadic from microsatellite unstable tumors in Lynch syndrome. Although BRAF mutations are common among sporadic colorectal cancers with MSI, they




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree