Complication
Incidence
Reference(s)
Hypocalcemia (HBS)
Primary
5–100 % (0–75 %)
Secondary
80–100 % (27–95 %)
RLN injury
Initial
0.1–1.5 %
Reoperation
2.3–6 %
Wound
Hematoma
< 1 %
Infection
0.02–0.3 %
Other
VTE
0.1–0.25 %
Pseudogout
Rare
[59]
Cardiac
0.08–0.11 %
Pulmonary
0.13–0.29 %
Pneumothorax
Rare
Tetraplegia
Rare
[58]
Hyperthyroidism
Biochemical
33 %
[54]
Clinical
Rare
[55]
Similarly, the potential for hypocalcemia following parathyroid surgery was demonstrated in early clinical experience. The first “successful” parathyroidectomy was performed by Isaac Olch in 1929 [1, 3]. The patient had significant bone disease with cystic tumors and a palpable parathyroid gland. Postoperatively, the patient developed tetany and was treated with calcium. Postoperative hypocalcemia in these patients may be related to intentional or accidental removal or devascularization of all functional parathyroid tissue. In addition, the clinical picture may be contributed to by suppression of the remaining normal glands or by an abrupt change in the balance of bone remodeling. Prolonged, significant hypocalcemia due to this latter mechanism is referred to as hungry bone syndrome .
The presumed pathophysiology of hungry bone syndrome results from the impact of a prolonged period of excess parathyroid hormone (PTH) on the process of bone remodeling [7]. Crucial to normal bone health, remodeling consists of osteoclast-mediated bone resorption coupled 2–3 weeks later with osteoblast-mediated bone formation. The portion of bone in this “in-between” phase is referred to as the remodeling space. In the preoperative state, PTH elevation tips the balance in favor of osteoclastic resorption resulting in an expansion of the remodeling space and an overall depletion in bone mineralization. Following successful parathyroidectomy, bone resorption abruptly decreases tipping the balance in favor of formation. The drop in remodeling space requires increased skeletal usage of minerals and may result in the abrupt drop in plasma levels of calcium and phosphate. While most hypocalcemia following surgery is mild and self-limiting, the term hungry bone syndrome is typically reserved for those requiring significant supplementation, often in the form of intravenous (IV) calcium over a prolonged period of time.
Hypocalcemia may present with perioral or extremity paresthesias and/or muscle cramping. Clinical assessment can be performed with Chvostek’s or Trousseau’s signs [8] . Chvostek’s sign is elicited by tapping over the proximal facial nerve with resultant muscle twitching at the corner of the mouth. While simple to perform, this test is lacking in both specificity, as it is present in 10 % of the normal population, and sensitivity, being absent in 30 % of those with hypocalcemia [9, 10]. The Trousseau sign is tested by inflating a blood pressure cuff above the systolic pressure for 2–3 min. The resultant hypoxia and acidosis are then observed to elicit carpo-pedal spasm in the setting of hypocalcemia -related nerve irritability. This test is more sensitive than Chvostek’s but is more arduous and painful to perform. Thus, some authors have suggested that its practice be abandoned [8]. More profound hypocalcemia can result in seizures, tetany, and in rare cases cardiac failure, which is reversible with treatment [11, 12].
Hypocalcemia: Primary Hyperparathyroidism
Reported rates of hypocalcemia following surgery for primary hyperparathyroidism vary widely in the literature. This likely reflects the heterogeneity in patients, the duration of disease, and surgical practice across publications. Early reports of transient postoperative hypocalcemia in the 1980s quoted rates in the 20–50 % range [7, 13, 14]. The higher side of this range appeared to be associated with the practice of routine biopsy of all parathyroids found during bilateral exploration. While larger contemporary series tend to quote lower rates, a wide range continues to be reported in the literature. This variability appears to be due to a wide number of factors related to: (1) the patient, (2) the disease, and (3) surgical findings and techniques (Table 2).
Table 2
Factors predicting hypocalcemia after parathyroidectomy
Factor | ↑/↓ | References |
|---|---|---|
Primary hyperparathyroidism | ||
Age | ↑(↓) | |
Gender | No effect | [18] |
Preoperative calcium | ↑ | |
Preoperative PTH | ↑ | |
Preoperative vitamin D | ↑ | |
Bone disease | ↑ | |
Preoperative ALP | ↑ | |
Calcium-lowering meds | ↑ | [19] |
Extensive exploration/bx | ↑ | |
# Glands removed | ↑ | |
Adenoma weight | ↑ | |
PTH drop > 85 % | ↑ | |
Secondary hyperparathyroidism | ||
Age | ↓ | [39] |
Pre-op calcium | ↓ | [41] |
Pre-op ALP | ↓ | [41] |
Pre-op PTH | ↑ | [42] |
Calcium-lowering meds | ↑ | [40] |
Weight removed | ↓ | [41] |
Conflicting reports exist regarding the impact of age on the risk of postoperative hypocalcemia. Some reports have found an association with older age [15, 16]. Conn et al. found that postoperative hypocalcemia was significantly more common in older patients and in those with hypertension [16]. In contrast, Zuberi et al. reported that younger patients seem to be at higher risk [17]. Gender, however, does not appear to have a significant, independent impact [18]. Preoperative medications have also been linked to the risk of postoperative hypocalcemia . Schnieder et al. reported a series of 281 patients undergoing surgery for primary hyperparathyroidism [19]. On multivariate analysis, the authors found that patient taking two or more calcium-lowering medications were significantly more likely to encounter postoperative hypocalcemia.
The role of the patient’s vitamin D status has also been suggested to influence postoperative calcium levels. Stewart et al. reported a higher incidence of symptomatic hypocalcemia following parathyroid surgery in vitamin D-deficient patients [20]. Lang et al. noted similar findings in a series of 80 patients treated with minimally invasive parathyroidectomy [21]. However, the authors noted that these patients also tended to have a higher preoperative PTH bringing into question the mechanism of this effect. In contrast, Press et al. in a series of more than 1700 patients noted no difference in oral calcium intake and postoperative, symptomatic hypocalcemia between groups of patients with markedly different vitamin D levels. Potentially, the routine use of supplementation in these patients may have masked smaller differences. In all three aforementioned series, 25-hydroxy vitamin D3 was measured.
As one might expect, certain disease factors seem to be linked to the risk of postoperative hypocalcemia. Higher levels of both PTH and calcium have been linked to increased risk [16]. Using multivariate analysis on a series of 6000 patients undergoing parathyroidectomy for primary hyperparathyroidism , Vasher et al. found preoperative calcium was independently predictive of postoperative hypocalcemia [22]. At their center, the authors utilize the preoperative calcium level as part of the protocol to determine upfront the level of post parathyroidectomy calcium supplementation. Similarly, Lang et al. found preoperative PTH levels to be significantly higher in patients requiring postoperative calcium supplementation [21]. These findings are certainly not universal with several additional series failing to observe a significant link between preoperative laboratory values and hypocalcemia in the postoperative period [17, 20, 23–25].
Given the proposed role of increased skeletal mineral usage in the etiology of postoperative hypocalcemia, it would seem reasonable to hypothesize that more advanced bone disease would portend a higher risk. Certainly, series that report a substantial rate of skeletal manifestations tend to have high rates of postoperative hypocalcemia [26, 27]. Gopal et al. reported a series of 79 patients treated for primary hyperparathyroidism in western India [27]. In this patient population, radiographic evidence of skeletal manifestations was evident in 75 % of the patients. In this series, all patients were started on immediate postoperative oral calcium. Despite this, 60 % of patients developed transient hypoparathyroidism and 30 % showed evidence of significant hungry bone syndrome. Similarly, Agarwal et al. reported symptomatic hypocalcemia despite routine oral calcium and vitamin D supplementation in 46/52 patients with osteitis fibrosa cystica following surgery. All 46 patients required treatment with IV calcium gluconate. In comparison, large studies from more developed countries in which most patients have asymptomatic hyperparathyroidism and advanced bone disease is rare report significantly lower rates of hypocalcemia and rarely mention IV calcium infusion [22, 28, 29].
Radiologic evidence of skeletal manifestations may include osteitis fibrosa cystica, lytic lesions, browns tumors, subperiosteal erosions, and fractures [7, 26, 27] (Fig. 1) In addition, Vasher et al. found that a bone mineral density t score of < 3.0 was predictive of postoperative hypocalcemia on multivariate analysis. Alkaline phosphatase has also been suggested as a serum marker of more advanced bone disease. Multiple series have demonstrated that elevated levels of alkaline phosphatase were associated with the need for postoperative calcium [15, 30]. Additional serum bone markers are not used in routine clinical practice.
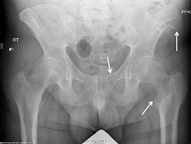

Fig. 1
Radiograph showing advanced bone disease with osteitis fibrosa cystica in a patient with hyperparathyroidism. Arrows indicate osteolytic bone lesions
Intraoperative factors have also demonstrated a clear impact. In general, hypocalcemia has been associated with more extensive removal or manipulation of parathyroid tissue [13, 14, 16, 17, 22, 24, 25, 31, 32]. The practice of routine biopsy of all parathyroid glands during a bilateral exploration was largely abandoned in the 1980s after it was shown to result in higher rates of postoperative hypocalcemia with similar cure rates compared to a more conservative strategy [13]. Even in the absence of biopsies, it appears that more extensive exploration may result in a greater degree of hypoparathyroidism [16, 22, 31, 32]. In a randomized controlled trial of unilateral versus bilateral exploration, Bergenfelz et al. reported that patients undergoing the more extensive operation required more oral calcium supplementation, and had lower postoperative calcium levels and more frequent episodes of symptomatic hypocalcemia [31]. In a subsequent review of a large-scale national database, including more than 2700 patients, the same author demonstrated lower rates of postoperative hypoparathyroidism associated with the use of preoperative localization and intraoperative PTH [32].
Given the aforementioned reports, it is not surprising that postoperative hypocalcemia is more common when a greater number of glands require removal at surgery [17, 22, 24]. Even when a single gland is removed, the weight of the parathyroid adenoma appears to be correlated with the need for calcium supplementation [7, 14, 15]. In addition, the magnitude of the drop in intraoperative PTH is predictive of the postoperative course [23, 33]. Crea et al. found that an 85 % drop in intraoperative PTH was reliably predictive of postoperative hypocalcemia in patients with primary hyperparathyroidism .
Perhaps the final factor contributing to the wide range of quoted rates in the literature lies in the evolution and variability of postoperative care. In particular, there has been a shift toward short-stay or same-day discharge after parathyroid surgery in many centers. As postoperative issues have the potential to delay discharge, many centers now practice routine calcium supplementation following parathyroidectomy. In the series of 6000 patients reported by Vasher et al., routine postoperative oral calcium supplementation was tailored to the patients’ risk for postoperative hypocalcemia [22]. In that report, less than 8 % of patients reported hypocalcemic symptoms and just 0.1 % required return to the emergency department for IV calcium. Thus, it is likely that oral supplementation is given to many patients in sufficient quantities to avoid hypocalcemia that would have been reported in earlier series during which this practice was less common.
Permanent hypoparathyroidism is exceedingly rare (< 1 %) following initial surgery. While significantly higher rates have been reported following multiple explorations, more recent studies have demonstrated that reoperative parathyroid surgery in experienced hands can be performed with minimal morbidity [34–37].
Hypocalcemia: Renal Hyperparathyroidism
Rates of hypercalcemia following surgery for renal hyperparathyroidism are significantly higher than those seen in the primary hyperparathyroidism literature [24, 38–42]. Certainly, this should not come as a surprise when the initial etiology in this population is linked to hypocalcemia. Virtually all patients undergoing operation for secondary hyperparathyroidism require postoperative oral calcium supplementation. In addition, more profound hypocalcemia related to hungry bones is significantly more common. Goldfarb et al. reported that 27 % of 79 patients undergoing subtotal parathyroidectomy for secondary hyperparathyroidism required postoperative IV calcium [39].
Similar to the literature on primary hyperparathyroidism , several authors have examined the risk factors for significant postoperative hypocalcemia and hungry bone syndrome following parathyroidectomy. Contrary to the aforementioned literature, young age and low preoperative calcium and alkaline phosphatase levels appear to be linked to the development of severe hypocalcemia. The data on preoperative PTH are less clear with some reports showing no link [39–41] while others associate a high PTH with postoperative hypocalcemia [42]. Preoperative use of cinecalcet has also been linked to hungry bone syndrome [40]. Interestingly, Torer et al. reported that lower weight of resected parathyroid tissue was associated with postoperative hypocalcemia opposite of what has been observed in primary hyperparathyroidism [41].
Postoperative Care
At our institution, patients are followed up postoperatively with twice-daily serum calcium (albumin corrected) and phosphate levels. Magnesium levels are obtained in cases of difficult-to-correct hypocalcemia or suspected hungry bone disease. Routine oral calcium supplementation is not routinely given in cases of primary hyperparathyroidism as we do not practice same-day discharge. Oral calcium supplementation may be started at the surgeons’ discretion if a high likelihood of significant postoperative hypocalcemia is suspected. Mild postoperative hypocalcemia in patients with primary hyperparathyroidism is typically manageable with oral calcium and vitamin D supplementation alone. Patients with secondary hyperparathyroidism are followed up for a longer period of time, as patients with hungry bone syndrome may not reach the nadir of their serum calcium level for several days after surgery [39]. The patients are placed on oral calcium supplementation and resume their 1,25-dihydroxy vitamin D3 supplements. Close coordination with the dialysis team is important as alterations of calcium concentration in the dialysate are often utilized to control serum levels in those with hungry bone syndrome. When required, IV calcium gluconate infusion is instituted. Prolonged infusions or more concentrated solutions are given through a peripherally inserted central catheter (PICC) or central venous line.
Hypocalcemia Summary
Considerable variability in the rates of postoperative hypocalcemia is quoted in the literature. Significant, prolonged hypocalcemia is uncommon in primary hyperparathyroidism without bone involvement. When this does occur, it can typically be managed with oral calcium supplementation. In contrast, hypocalcemia is more common following surgery for secondary hyperparathyroidism. These patients are more likely to require IV calcium infusion and more prolonged in-hospital observation. Specific risk factors have been identified which increase the risk of significant hypocalcemia following surgery. Many of these risk factors are specific to either primary or secondary disease.
Other Complications
RLN Injury
Injury to the recurrent laryngeal nerve (RLN) is uncommon in parathyroid surgery. Nonetheless, careful dissection is warranted given the proximity of the parathyroid glands to the nerve. Untch et al. examined the proximity of the parathyroid glands to the RLN in 136 patients undergoing parathyroid surgery. The authors noted the median distance to the nerve was 5 mm, with the superior glands on average lying closer than the inferior glands [43]. Large series from centers with experienced endocrine surgeons typically report rates between 0 and 1.5 % for initial surgery [8, 28, 29]. However, the association between surgeon experience and complications in endocrine surgery has been well documented and may limit the generalizability of these findings [44]. Early studies suggested that reoperative exploration carried a significantly higher risk. Patow et al. reported a 6.6 % rate of nerve injury in 163 patients undergoing reoperative surgery [45]. However, more recent series, utilizing preoperative localization algorithms and performed by experienced parathyroid surgeons, have reported injury rates closely approximating those seen in initial surgery [35, 36, 46].
Wound Complications
Postoperative hematoma is a rare complication of parathyroid surgery. Most series quote rates of less than 1 % [28, 47, 48]. Regardless, this complication is potentially life-threatening and should be managed with expedient exploration and evacuation. Similarly, surgical site infection is quite rare after parathyroid/thyroid surgery. Gupra et al. examined 30-day mortality and morbidity following thyroid and parathyroid surgeries using the National Surgical Quality Improvement Program database over a 2-year period. In a sample of 6154 parathyroid surgeries, superficial and deep neck infections were seen in just 0.3 and 0.02 %, respectively [49]. As parathyroid surgery is considered a “clean” procedure, routine preoperative antibiotic prophylaxis is not mandated [50]. In a randomized, controlled trial of 500 patients undergoing thyroidectomy, Aveia et al. failed to show any difference in surgical site infection between those given routine antibiotic prophylaxis and those who were not [51]. While evidence does not exist to support the routine use of antibiotics in these operations, practice appears to vary between centers and surgeons. Moalem et al. surveyed 518 members of the American Association of Endocrine Surgeons (AAES) and the International Association of Endocrine Surgeons (IAES) [50]. Of the 57.1 % who responded, 62 % reported that they “never” administer preoperative antibiotics and 26.2 % responded that they “always” do.
Rare Complications
Additional complications have been reported following parathyroid surgery but are exceedingly rare. Deep venous thrombosis and pulmonary embolism are seen in 0.1–0.25 % [29, 49, 52]. Roy et al. compared the incidence of deep vein thrombosis/pulmonary embolism (DVT/PE) in 16,022 patients who underwent thyroid and parathyroid surgery with a total cohort of almost 350,000 patients undergoing any surgical procedure [52]. They found that the risk of DVT/PE (0.16 %) was significantly lower than in the larger population of surgical patients. In addition, given that the likelihood of return to the operating room for a neck hematoma was ten times that of DVT/PE, the authors recommended that pharmacologic prophylaxis be reserved for patients at higher risk of venous thromboembolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree