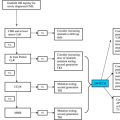
Venous thrombosis results from the convergence of vessel wall injury and/or venous stasis, known as local triggering factors, and the occurrence of acquired and/or inherited thrombophilia, also known as systemic prothrombotic risk factors. Portal vein thrombosis (PVT) and Budd–Chiari syndrome (BCS) are caused by thrombosis and/or obstruction of the extrahepatic portal veins and the hepatic venous outflow tract, respectively. Several divergent prothrombotic disorders may underlie these distinct forms of large vessel thrombosis. While cirrhotic PVT is relatively common, especially in advanced liver disease, noncirrhotic and nontumoral PVT is rare and BCS is of intermediate incidence. In this article, we review pathogenic mechanisms and current concepts of patient management.
Portal vein thrombosis (PVT) and Budd–Chiari syndrome (BCS) are caused by thrombosis and/or obstruction of the extrahepatic portal veins and the hepatic venous outflow tract, respectively. Several heterogeneous prothrombotic disorders may cause thrombosis of the portal and hepatic veins. Venous thrombosis usually results from the convergence of vessel wall injury and/or venous stasis, known as local triggering factors, and the occurrence of acquired and/or inherited thrombophilia, also known as systemic prothrombotic risk factors.
Risk factors for portal vein thrombosis and Budd–Chiari syndrome
Local risk factors are responsible for 30% to 40% of the cases of PVT, but they are rarely reported in subjects with primary BCS. Inflammatory intra-abdominal disorders such as appendicitis and pancreatitis, postoperative complications of abdominal surgery, particularly splenectomy and surgical portosystemic shunts, and portal hypertension are recognized intra-abdominal risk factors for PVT ( Table 1 ). In contrast, distinct systemic prothrombotic risk factors have been recognized in 60% to 70% of PVT and up to 90% of BCS ( Table 2 ). All of these have been associated with an increased systemic predisposition to deep vein thrombosis and/or pulmonary embolism and more than one prothrombotic risk factor has been implicated in the pathogenesis of PVT and BCS in up to one third of the patients.
| Portal Vein Thrombosis | Budd–Chiari Syndrome |
|---|---|
|
|
| Prothrombotic Disorders | PVT, % a | BCS, % b | DVT, % c | Healthy Subjects, % c |
|---|---|---|---|---|
| Myeloproliferative diseases d | 14–35 | 28–47 | NA | NA |
| Antiphospholipid syndrome | 5–23 | 5–21 | 4–21 | 5 |
| Factor V Leiden mutation | 3–14 | 14–31 | 15–20 | 5–12 |
| Factor II gene mutation | 3–22 | 4–6 | 4–8 | 1 |
| Protein C deficiency | 0–9 | 0–13 | 3–6 | 0.2–0.5 |
| Protein S deficiency | 2–30 | 0–6 | 2 | 0.03–0.13 |
| Antithrombin deficiency | 0–4.5 | 0–4 | 0.5–7.5 | 0.02 |
| C677T MTHFR gene mutations e | 0–11 | 13–52 | Variable | 12–46 |
| Hyperhomocysteinemia | NA | 0–37 | 10–25 | 5–10 |
| Elevated factor VIII | NA | NA | 15–25 | NA |
| Pregnancy | 0–4 | 0–15 | f | NA |
| Oral contraceptive use | 0–48 | 7–55 | f | NA |
| None | 16–22 | 6–23 | 50 | NA |
a Adapted from Refs. 8–11,53,57
b Adapted from Refs. 9,10,12–15,36
d Occult MPD was not investigated in most of the cohorts.
e The presence of C677T MTHFR gene mutations without an increase in homocystein level is not a risk factor for DVT.
f Accounts for a threefold increase in the relative risk for DVT.
Some risk factors are more frequently associated with one or another of those vascular disorders of the liver. In this respect, most of the cases of PVT are attributable to Philadelphia (Ph) chromosome negative myeloproliferative diseases (MPD), antiphospholipid syndrome, or distinct inherited prothrombotic disorders, such as protein S deficiency and prothrombin gene mutation (see Table 2 ). There is a great geographic variation, but MPD is one of the most frequent disorders associated with PVT. In general, the prevalence of inherited thrombophilia is far less common in the East when compared with the West.
Local risk factors account for only a small number of the cases of BCS, which can then be designated as “secondary” BCS (see Table 1 ). Most patients with BCS have a “primary” disorder and share one or more prothrombotic risk factors, mainly MPD, oral contraceptive use, factor V Leiden mutation, and antiphospholipid syndrome. Myeloproliferative disorders, particularly polycythemia vera, are observed in approximately half of the cases of BCS, but fewer than 10% of the subjects with overt MPD develop BCS. There is also some geographic variation in the prevalence of acquired thrombophilia in patients with BCS. The use of oral contraceptives is related to BCS more frequently in Western, when compared with Eastern patients. On the other hand, poor sanitation has been associated with BCS, particularly with inferior vena cava (IVC) involvement in the Asian countries and poor socioeconomic status.
Evaluation of thrombophilia, portal vein thrombosis, and Budd–Chiari syndrome
Because of the high frequency of inherited and/or acquired prothrombotic disorders in patients with PVT and BCS, a systematic investigation of these risk factors is warranted and a formal hematological consultation is usually recomended, because the usual diagnostic criteria for those disorders cannot always be applied to subjects with underlying liver disease.
Myeloproliferative diseases are the single most common group of disorders associated with PVT and BCS. In most cases, the Ph chromosome negative MPD can be classified as polycythemia vera, essential thrombocythemia or myelofibrosis. These diagnoses in patients with PVT and BCS, using the current World Health Organization criteria, may be misleading because of the presence of increased plasma volume and hypersplenism in the subjects with chronic liver disease (CLD) and portal hypertension. In this regard, detection of spontaneous endogenous erythrocyte colonies (EEC), determination of the somatic JAK2 V617F mutation, and bone marrow biopsy to assess the presence of dystrophic megakariocytes has been used to diagnose MPD without typical phenotypic markers in subjects with and without CLD. Based on the results of these assays, up to two thirds of the patients with either PVT or BCS were shown to have either overt or occult MPD. However, some drawbacks associated with use of these parameters to diagnose occult MPD have to be highlighted.
The EEC assay is a nonstandardized labor-intensive method that is highly dependant on local laboratory expertise. It can yield negative results in subjects with clear-cut criteria for MPD and positive results in healthy subjects and patients with nonclonal polycythemia, which limits its diagnostic accuracy for MPD.
Recently, several investigators have identified an acquired mutation in the autoregulatory pseudokinase domain (JH2) of Janus kinase-2 (JAK2) gene in patients with MPD. One substitution of valine to phenylalanine at position 617 (V617F) was observed in 90% to 95% of the patients with polycythemia vera, 50% to 70% of the patients with essential thrombocythemia, and 40% to 50% of the patients with myelofibrosis. Preliminary data have also suggested that MPD patients with JAK2 have an increased risk of thrombosis, hemorrhage, fibrosis, and cytoreductive treatment requirement when compared with their JAK2 negative counterparts ; however, data on the functional consequences of this mutation are still scarce. Noninvasive assessment of V617F JAK2 mutation for evaluation of MPD in patients with hepatic or splanchnic vein thrombosis is promising, but it should be emphasized that its presence is not sufficient for defining the MPD phenotype and that V617F JAK2 mutation may be absent in a significant proportion of patients with PVT and BCS with overt MPD.
Antiphospholipid antibodies syndrome (APS) is seen in 5% to 23% of the patients with PVT or BCS. The antiphospholipid antibodies are a group of autoantibodies that target phospholipid binding proteins, including lupus anticoagulant, IgM and IgG anticardiolipin, and antiβ2-glicoprotein I antibodies. As the presence of low-titer anticardiolipin is frequent in subjects with CLD, the diagnostic criteria for APS in such patients requires, apart from past evidence of thrombosis or miscarriages, the detection of one of those antibodies in medium or high titers two times at least 12 weeks apart.
Inherited deficiencies of antithrombin and proteins S and C are responsible for 15% to 30% of the cases of PVT and BCS. However, their diagnoses are sometimes challenging in patients with liver disease, because low protein levels could be a result of impaired hepatic synthesis and not related to inherited deficiency. In accordance with this assumption is the reversal of coagulation factor abnormalities after surgical treatment of extrahepatic portal vein obstruction (EHPVO) by mesenteric to left portal vein bypass. In practice, deficiency of proteins S and C and antithrombin are assumed when no other abnormality in the coagulation factors levels is disclosed, indicating preserved liver function. Family studies could also be useful in doubtful cases.
Several other divergent situations deserve mention in this section. Increased levels of factor VIII have also been described in patients with BCS and PVT. However, as factor VIII is an acute phase protein, its increase could reflect liver disease per se and not inherited thrombophilia. Diagnosis has to rely on the determination of factor VIII levels as well as other acute phase proteins in different time points. Family studies could also be useful in this situation. In contrast, prothrombin G20210A gene mutation and factor V G1691A gene variant are frequently encountered in this setting. Their diagnosis is straightforward based on polymerase chain reaction (PCR)-based assays. However, the role of high homocysteine levels in thrombosis is controversial. Hyperhomocysteinemia can be detected by high performance liquid chromatography or chemiluminescence. Testing solely for methylene tetrahydrofolate reductase (MTHFR) C677T gene mutation is unreliable since heterozygosity and homozygosity for MTHFR C677T variant is observed in 50% and 10% of the healthy population, respectively. Paroxysmal nocturnal hemoglobinuria and Behcet’s disease may also be observed, particularly in subjects with BCS.
Based on recommendations from an expert panel, the investigation of thrombophilia in patients with BCS and PVT should initially include complete blood cell count, assays for plasma levels of coagulation factors and inhibitors, determination of genetic factor V and prothrombin gene mutations, assessment of antiphospholipid antibodies and lupus anticoagulant, and flow cytometry testing for paroxysmal nocturnal hemoglobinuria. Bone marrow biopsy, determination of blood cell mass and serum erythropoietin, as well as EEC, and the recently discovered JAK2 mutation should be the next steps in the evaluation of MPD ( Table 3 ).
| Myeloproliferative disorders |
|
| Paroxysmal nocturnal hemoglobinuria | Flow cytometry for CD55- and CD59-deficient cells |
| Antiphospholipid syndrome | Lupus anticoagulant, a IgM and IgG anticardiolipin antibodies, antib2-glicoprotein I and antinuclear factors |
| Factor V Leiden mutation | Detection of G1691A gene variant by PCR-based methods |
| Prothrombin gene mutation | Detection of G20210A gene mutation by PCR-based methods |
| Protein C, S, and Antithrombin III | Functional assays in the presence of normal clotting factor levels plasma levels a |
| Hyperhomocysteinemia | Blood folate, vitamin BI2 and homocysteine levels. Search for MTHFR polymorphism only when the level of homocysteine is high |
a Levels are influenced by anticoagulation; testing for multiple abnormalities is advisable, because several patients with BCS and portal vein thrombosis had one or more overlapping prothrombotic risk factors.
Evaluation of thrombophilia, portal vein thrombosis, and Budd–Chiari syndrome
Because of the high frequency of inherited and/or acquired prothrombotic disorders in patients with PVT and BCS, a systematic investigation of these risk factors is warranted and a formal hematological consultation is usually recomended, because the usual diagnostic criteria for those disorders cannot always be applied to subjects with underlying liver disease.
Myeloproliferative diseases are the single most common group of disorders associated with PVT and BCS. In most cases, the Ph chromosome negative MPD can be classified as polycythemia vera, essential thrombocythemia or myelofibrosis. These diagnoses in patients with PVT and BCS, using the current World Health Organization criteria, may be misleading because of the presence of increased plasma volume and hypersplenism in the subjects with chronic liver disease (CLD) and portal hypertension. In this regard, detection of spontaneous endogenous erythrocyte colonies (EEC), determination of the somatic JAK2 V617F mutation, and bone marrow biopsy to assess the presence of dystrophic megakariocytes has been used to diagnose MPD without typical phenotypic markers in subjects with and without CLD. Based on the results of these assays, up to two thirds of the patients with either PVT or BCS were shown to have either overt or occult MPD. However, some drawbacks associated with use of these parameters to diagnose occult MPD have to be highlighted.
The EEC assay is a nonstandardized labor-intensive method that is highly dependant on local laboratory expertise. It can yield negative results in subjects with clear-cut criteria for MPD and positive results in healthy subjects and patients with nonclonal polycythemia, which limits its diagnostic accuracy for MPD.
Recently, several investigators have identified an acquired mutation in the autoregulatory pseudokinase domain (JH2) of Janus kinase-2 (JAK2) gene in patients with MPD. One substitution of valine to phenylalanine at position 617 (V617F) was observed in 90% to 95% of the patients with polycythemia vera, 50% to 70% of the patients with essential thrombocythemia, and 40% to 50% of the patients with myelofibrosis. Preliminary data have also suggested that MPD patients with JAK2 have an increased risk of thrombosis, hemorrhage, fibrosis, and cytoreductive treatment requirement when compared with their JAK2 negative counterparts ; however, data on the functional consequences of this mutation are still scarce. Noninvasive assessment of V617F JAK2 mutation for evaluation of MPD in patients with hepatic or splanchnic vein thrombosis is promising, but it should be emphasized that its presence is not sufficient for defining the MPD phenotype and that V617F JAK2 mutation may be absent in a significant proportion of patients with PVT and BCS with overt MPD.
Antiphospholipid antibodies syndrome (APS) is seen in 5% to 23% of the patients with PVT or BCS. The antiphospholipid antibodies are a group of autoantibodies that target phospholipid binding proteins, including lupus anticoagulant, IgM and IgG anticardiolipin, and antiβ2-glicoprotein I antibodies. As the presence of low-titer anticardiolipin is frequent in subjects with CLD, the diagnostic criteria for APS in such patients requires, apart from past evidence of thrombosis or miscarriages, the detection of one of those antibodies in medium or high titers two times at least 12 weeks apart.
Inherited deficiencies of antithrombin and proteins S and C are responsible for 15% to 30% of the cases of PVT and BCS. However, their diagnoses are sometimes challenging in patients with liver disease, because low protein levels could be a result of impaired hepatic synthesis and not related to inherited deficiency. In accordance with this assumption is the reversal of coagulation factor abnormalities after surgical treatment of extrahepatic portal vein obstruction (EHPVO) by mesenteric to left portal vein bypass. In practice, deficiency of proteins S and C and antithrombin are assumed when no other abnormality in the coagulation factors levels is disclosed, indicating preserved liver function. Family studies could also be useful in doubtful cases.
Several other divergent situations deserve mention in this section. Increased levels of factor VIII have also been described in patients with BCS and PVT. However, as factor VIII is an acute phase protein, its increase could reflect liver disease per se and not inherited thrombophilia. Diagnosis has to rely on the determination of factor VIII levels as well as other acute phase proteins in different time points. Family studies could also be useful in this situation. In contrast, prothrombin G20210A gene mutation and factor V G1691A gene variant are frequently encountered in this setting. Their diagnosis is straightforward based on polymerase chain reaction (PCR)-based assays. However, the role of high homocysteine levels in thrombosis is controversial. Hyperhomocysteinemia can be detected by high performance liquid chromatography or chemiluminescence. Testing solely for methylene tetrahydrofolate reductase (MTHFR) C677T gene mutation is unreliable since heterozygosity and homozygosity for MTHFR C677T variant is observed in 50% and 10% of the healthy population, respectively. Paroxysmal nocturnal hemoglobinuria and Behcet’s disease may also be observed, particularly in subjects with BCS.
Based on recommendations from an expert panel, the investigation of thrombophilia in patients with BCS and PVT should initially include complete blood cell count, assays for plasma levels of coagulation factors and inhibitors, determination of genetic factor V and prothrombin gene mutations, assessment of antiphospholipid antibodies and lupus anticoagulant, and flow cytometry testing for paroxysmal nocturnal hemoglobinuria. Bone marrow biopsy, determination of blood cell mass and serum erythropoietin, as well as EEC, and the recently discovered JAK2 mutation should be the next steps in the evaluation of MPD ( Table 3 ).
| Myeloproliferative disorders |
|
| Paroxysmal nocturnal hemoglobinuria | Flow cytometry for CD55- and CD59-deficient cells |
| Antiphospholipid syndrome | Lupus anticoagulant, a IgM and IgG anticardiolipin antibodies, antib2-glicoprotein I and antinuclear factors |
| Factor V Leiden mutation | Detection of G1691A gene variant by PCR-based methods |
| Prothrombin gene mutation | Detection of G20210A gene mutation by PCR-based methods |
| Protein C, S, and Antithrombin III | Functional assays in the presence of normal clotting factor levels plasma levels a |
| Hyperhomocysteinemia | Blood folate, vitamin BI2 and homocysteine levels. Search for MTHFR polymorphism only when the level of homocysteine is high |
a Levels are influenced by anticoagulation; testing for multiple abnormalities is advisable, because several patients with BCS and portal vein thrombosis had one or more overlapping prothrombotic risk factors.
Portal vein thrombosis
Extrahepatic obstruction of the portal vein can be caused by thrombosis or by compression or occlusion of the portal trunk or one of its branches by tumors, particularly hepatocellular carcinoma. In autopsy studies, PVT was found in approximately 1% of the cases, most of them related to cirrhosis or liver cancer with less than one third of the cases attributable to noncirrhotic, nonmalignant PVT. To better characterize those patients without malignancy, two recent consensus conferences have recommended the term EHPVO to encompass not only the subjects with recent PVT, but also those with the chronic form known as portal cavernoma. The panel also suggested the categorization as distinct subgroups the cases of intrahepatic PVT and PVT in association with cirrhosis. The following sections address several types of EHPVO beginning with that observed in childhood and concluding with cirrhosis-associated forms of this disorder.
Extrahepatic Portal Vein Obstruction in Childhood
The frequency of EHPVO is higher in Asia and South America, where it affects mainly children. Most of the cases of EHPVO in childhood are either idiopathic or associated with a past history of umbilical sepsis or umbilical vein catheterization. Even though inherited or acquired thrombophilia were reported to be rare in children with EHPVO, one Egyptian study has described the association of one or more inherited prothrombotic disorders in up to 63% of the children with EHPVO.
Clinical manifestations in children are mainly a result of portal hypertension. Most have chronic EHPVO with portal cavernoma disclosed by ultrasound (US) or by MRI or CT angiogram. Variceal bleeding is the most common presentation, but cholestasis caused by portal biliopathy (biliary abnormalities including strictures as a result of extrinsic bile duct compression by dilated venous collaterals), growth retardation, and abdominal distension as a result of splenomegaly or pancytopenia caused by hypersplenism may also occur at presentation. The natural history of EHPVO is usually benign. However, some patients may develop signs of liver failure, which may be ascribed to concurrent hepatitis B or C or liver dysfunction as a result of parenchymal extinction. Management of EHPVO in children is based on uncontrolled studies and includes sclerotherapy or endoscopic band ligation (EBL) for the control of acute variceal bleeding and EBL and/or beta-blockers for secondary prophylaxis.
Anticoagulation should be restricted to those subjects with recognizable prothrombotic disorders. Portal biliopathy treatment is advised only for symptomatic cases, preferably by endoscopic therapy. Surgical shunts, particularly distal splenorenal shunts, have been performed for variceal bleeding or portal biliopathy refractory to endoscopic therapy, but the use of mesenteric to left portal vein bypass (Rex shunt) has been advocated to be more physiologic, as it restores portal venous blood flow to the liver via the left portal vein in the Rex recessus. The use of Rex shunts has been associated with excellent long-term outcomes including no further bleeding episodes from varices, partial or complete regression of splenomegaly, improvement in platelet and leukocyte counts, and normalization of coagulation abnormalities in most of the surgically treated subjects. These findings altogether have led some authorities to consider this surgical procedure as the therapy of choice for symptomatic EHPVO in children, when technically feasible.
Nonmalignant, Noncirrhotic Extrahepatic Portal Vein Obstruction in Adults
Nontumoral EHPVO in adults can be classified according to presentation as acute PVT or chronic EHPVO, as well as to the presence or absence of cirrhosis, which is discussed separately in the next section. Portal vein thrombosis can also occur in association with other liver diseases caused by prothrombotic disorders such as BCS or noncirrhotic portal hypertension (NCPH). In adults, noncirrhotic, nontumoral EHPVO is quite rare and accounts for less than 10% of the cases of portal hypertension with fewer than 400 patients reported from three reference centers in Europe.
Clinical findings and management options vary according to the type of EHPVO. Acute or recent PVT is characterized by recent occlusion of the portal vein by a thrombus that can be associated or not with symptoms of abdominal pain and diarrhea, as well as signs of systemic inflammatory response or sepsis in case of pylephlebitis. Severity depends on the extent of involvement of the portal venous system. Bloody diarrhea, ascitis, and ileus are more common in severe superior mesenteric vein involvement that may lead to mesenteric ischemia with bowel perforation and septic shock. The diagnosis is usually performed by US, which shows hyperechoic material in the portal vein with downstream dilatation of the portal venous system with no flow detected by Doppler imaging ( Fig. 1 ).