© International Society of Gynecological Endocrinology 2015
Bart C. J. M. Fauser and Andrea R. Genazzani (eds.)Frontiers in Gynecological EndocrinologyISGE Series10.1007/978-3-319-09662-9_33. Polycystic Ovary Syndrome: From Contraception to Hormone Replacement Therapy
(1)
Department of Obstetrics and Gynecology, University of Pisa, Pisa, Italy
(2)
Department of Obstetrics and Gynecology, Gynecological Endocrinology Center, University of Modena and Reggio Emilia, Modena, Italy
3.1 Introduction
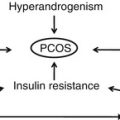
Polycystic ovary syndrome (PCOS) is a very peculiar disease and is one of the most frequent endocrine disorders in women as it occurs in as many as 8–10 % of women of reproductive age [1, 2]. For many years, there has been no agreement on the criteria on which to base the diagnosis of PCOS. This was probably a consequence of the heterogeneity of the syndrome, but also depended on the absence of clear pathogenetic mechanism(s) [3].
At first, the diagnostic criteria proposed by the NIH for PCOS were the presence of hyperandrogenism and chronic anovulation with clear exclusion of related ovulatory or other androgen excess disorders (i.e., hyperprolactinemia, thyroid diseases, androgen-secreting tumors and adrenal dysfunction/hyperplasia) [4]. These criteria did not include the presence of polycystic ovaries at ultrasound examination because it was observed that polycystic ovaries could also be present in healthy eumenorrheic women [5]. A few years later the diagnostic criteria were expanded and PCOS was considered to be present when at least two of the three features were diagnosed: oligo- or anovulation, clinical/biochemical hyperandrogenism, and polycystic ovaries as assessed by ultrasound examination [6]. This evolution was relevant because it permitted the inclusion of women with PCOS who had been excluded by previous criteria: those with polycystic ovaries affected by hyperandrogenism and ovulatory cycles, or chronic anovulation and normal androgen levels. After assessing this, we then have to clarify that PCOS is completely different from PCO. PCO means polycystic ovary and refers only to the morphological aspect of the ovary at ultrasound examination. Indeed, PCOS can be found in many other dysendocrinopathies such as hyperprolactinemia, thyroid dysfunction, and stress-induced amenorrhea.
3.2 Endocrine Profile of PCOS
Polycystic ovary syndrome is characterized by higher plasma concentrations of ovarian and adrenal androgens, increased luteinizing hormone (LH) levels, high estrogen levels (especially estrone) owing to extraglandular conversion from androgens, lower levels of sex hormone-binding globulin (SHBG) and higher levels of prolactin and insulin, the latter often in the presence of excess weight or obesity.
Although the pathogenesis of PCOS is still controversial [7–9], PCOS typically shows elevated LH and normal or relatively low follicle-stimulating hormone (FSH) secretion; thus, almost 50–60 % of PCOS patients show a high LH:FSH ratio (>2.5) [7, 8], an exaggerated LH response to gonadotropin-releasing hormone (GnRH) stimulation test [7, 8] and a higher frequency of LH pulsatile release from the pituitary [4, 7, 8, 10], which induces higher stimulation of theca cells and excess androgen secretion, as well as impaired follicular development [4].
Androgen excess is a classic feature of the syndrome, although it is not constant [7] and is a great part of ovarian production with an adrenal contribution, as a certain percentage of PCOS patients may show a mild steroidogenetic defect in the adrenal glands (such as for 21-hydroxylase) or merely greater adrenal hyperactivation owing to stress [11]. Androstenedione and testosterone are the best markers of ovarian androgen secretion, while dehydroepiandrosterone sulfate (DHEAS) is the best marker of adrenal secretion. Most testosterone is derived from peripheral conversion of androstenedione and from direct ovarian production. In addition, the adrenal glands contribute in part to testosterone, although in hyperandrogenic PCOS the main source of androgens is usually the ovaries. As cytochrome p450c17 is the androgen-forming enzyme in both the adrenal glands and the ovaries, whatever changes or increases its activity triggers the pathogenic mechanism underlying hyperandrogenism in PCOS [4]. In addition, in the presence of 5α-reductase, testosterone is converted within the cell to the more biologically potent androgen dihydrotestosterone. Excess or normal 5α-reductase activity in the skin determines the presence or absence of hirsutism [12]. Additionally, estrone plasma levels, a weak estrogen with biological activity 100 times less than estradiol, are increased as a result of the peripheral conversion of androstenedione by aromatase activity, which is more active in PCOS than in healthy controls, while estradiol levels are normal or low because of the frequent anovulatory cycles. All this results in a chronic hyperestrogenic state with the reversal of the estrone:estradiol ratio that may predispose to endometrial proliferation and to a possible increased risk of endometrial cancer [13, 14]. Another relevant aspect is the fact that normally less than 3 % of testosterone circulates as unbound in the serum. In fact, most circulating androgens are bound to SHBG, and thus biologically inactive. Any condition that decreases the levels of SHBG (such as an excess of circulating androgens) inducing reduced hepatic synthesis, leads to a relative excess of free circulating androgens. In PCOS, hirsutism usually occurs with decreased SHBG levels and obesity [4].
In addition, androgen excess may both directly and indirectly induce alterations in glucose metabolism, and ultimately be an additional cause of abnormal insulin sensitivity. Androgens may directly inhibit peripheral and hepatic insulin action. In fact, testosterone could induce insulin resistance in women with PCOS, acting on the post-binding signal, in particular by reducing the number and efficiency of glucose transport proteins, such as the type 4 glucose transporter (GLUT-4), especially in muscle and fat tissues [15]. It has also been reported that women with central obesity, typical of obese PCOS sufferers, have higher free androgen levels and exhibit significantly higher levels of insulin insensitivity than weight-matched controls and show increased free fatty acids [4].
3.3 PCOS, Brain and Abnormal Steroid Milieu
On the basis of what has been reported above, it is clear that in PCOS patients aesthetic problems such as acne, seborrhea and hirsutism can be terribly common and invalidating and their occurrence is simply related to the excess of androgens due to the abnormal ovarian function/regulation, which leads to chronic anovulation.
In most of these cases the only real solution is the use of a contraceptive pill. This choice is not merely related to the fact that normal menstrual cyclicity has to be re-established, but also to the fact that the clinical signs that PCOS patients show are invalidating, mainly from a psychological point of view. This aspect is quite complex; however, at its basis there are not only subjective complaints. but also greater vulnerability due to the impaired production of endogenous neurosteroids.
Indeed, it has been recently reported that patients suffering from menstrual disturbances (oligo- or amenorrhea) have a higher chance of experiencing changes in mood and behavior, anxiety and depression [16–18] and that such impairments are to a great extent related to the reduced ability to secrete active neurosteroids, such as allopregnanolone, inside the brain. Usually, estradiol, progesterone and DHEAS are the steroids that as inductors or substrate permit the regular synthesis of allopregnanolone. Whatever dysfunction of the ovarian function occurs, the steroid milieu is impaired and consequently the biosynthesis of neurosteroids to. Recently, we reported that in obese hyperinsulinemic PCOS patients, the absence of adrenal response in terms of allopregnanolone secretion to adrenocorticotropic hormone (ACTH) stimulation was restored under metformin administration [19], thus suggesting that on the basis of the frequently observed sexual dissatisfaction [16–18], as well as a high degree of occurrence of a depressive state [20] in PCOS, there is a lack of or an impaired synthesis of neurosteroids from the adrenal gland and/or by the brain. Moreover, being PCOS anovulatory in a higher rate with normal or abnormal menstrual cyclicity, high androgens and relatively low estrogens induce lower SHBG production, thus permitting a higher amount of free circulating androgens. On the other hand, low estrogens and no progesterone or very low progesterone due to anovulation determine low synthesis of neurosteroids in the brain.
It is quite clear that impairment of ovarian function affects various compartments and systems and the putative suggestion of the use of a contraceptive pill deserves consideration in blocking the abnormal ovarian function and reducing androgen production on the one hand and increasing the estrogenic milieu at the liver level, thus promoting SHBG synthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree