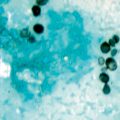
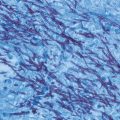
Fig. 14.1
Silver-stained bronchoalveolar lavage specimen showing characteristic clusters of Pneumocystis cysts
As noted above, the host-species specificity of Pneumocystis has led some to propose the division of Pneumocystis carinii into multiple unique species, with the nomenclature Pneumocystis jirovecii being used to refer to human P. carinii [7]. The proposal for a change in nomenclature has been questioned because it also calls for species distinction based on variation in gene sequences not known to result in a unique phenotype, so-called molecular phylogeny [8]. Pneumocystis that has not had a new name submitted for consideration can still be clearly defined using “special form” nomenclature (e.g., P. carinii f. sp. mustela for ferret P. carinii).
Epidemiology
PCP occurs only in patients who are significantly immunosuppressed, typically with abnormalities in CD4+ T lymphocytes or B cells. Serologic studies have demonstrated that a high proportion of the population has evidence of infection and that seroconversion typically occurs during childhood. A recent prospective longitudinal study demonstrated that seroconversion began in the first few months of life and by 20 months of age, 85 % of the infants in the study had seroconverted [9].
Aside from the serologic data, Pneumocystis was not known to actually infect the immunologically normal host. However, animal studies have proved that Pneumocystis produces a typical pattern of infection, transmission, and resolution in the normal host [10]. The other important biological feature of Pneumocystis infection is that the strain (or species) of Pneumocystis from any given mammalian host is transmissible only to members of the same host species. Cross-species transmission has never been convincingly demonstrated. Because of the finding of early seroconversion followed by disease later in life, PCP was postulated to be the result of reactivation of latent infection. However, no evidence for latency has ever been demonstrated, and mouse and rat models of PCP have shown that latency does not develop after infection. Considering all of these features, it would seem most likely that PCP is the result of new infection rather than reactivation of a latent infection. Person-to-person transmission is likely, based on the cumulative experience in animal models, but difficult to prove.
Without prophylaxis, PCP develops in approximately 70 % of adults and 40 % of infants and children with AIDS, and 10 % of patients with organ transplants. It is often the sentinel event identifying infants with severe congenital immunodeficiencies such as severe combined immunodeficiency (SCID) syndrome. PCP also is a frequent occurrence in patients being treated for malignancies, occurring with an overall frequency of 10–15 %. The actual incidence for any given malignancy depends on the treatment regimen and is positively correlated with the number of chemotherapeutic agents and the intensity of treatment.
Pathogenesis and Immunology
Control of infection is dependent on normally functioning CD4+ T lymphocytes. Studies in patients with AIDS show an increase in the occurrence of Pneumocystis pneumonia as CD4+ T lymphocytes drop. For adults and children over 6 years of age, a CD4+ T cell count of 200 cells/μl or lower is a marker of very high risk for the development of PCP. Based on the occurrence of PCP in some patients and mouse strains with various immunologic defects which result in defective antibody production, a possible role for CD4+ T lymphocytes could be to provide help for the production of specific antibody. Passively administered antibody has been shown to aid in the clearance of Pneumocystis in mouse models. Thus, antibody could be involved in the clearance of organisms through interaction with complement, phagocytes, and/or T lymphocytes.
The mechanism by which Pneumocystis damages the lung is not yet fully defined. Animal models have been valuable in helping us understand the immunopathogenesis of PCP [11]. Infection of SCID mice with Pneumocystis produces very little alteration in lung histology or function until very late in the course of the disease. However, if Pneumocystis-infected SCID mice are immunologically reconstituted with normal splenocytes, there is a rapid onset of an inflammatory response that results in an intense cellular infiltrate, markedly reduced lung compliance and significant hypoxia, all changes seen in humans with PCP. These inflammatory changes are associated with marked disruption of surfactant function. T cell subset analysis has shown that CD4+ T lymphocytes produce an inflammatory response that not only clears the organisms but also results in lung injury. In contrast, CD8+ T lymphocytes are ineffective in the eradication of Pneumocystis, but do produce a marked injurious inflammatory response, especially in the absence of CD4+ T lymphocytes.
Immune reconstitution inflammatory syndrome (IRIS), also called immune restitution disease or immune reconstitution syndrome, is a recently described manifestation of pulmonary infection in AIDS patients with Pneumocystis, Mycobacterium tuberculosis, and other pulmonary pathogens who are experiencing rapid reconstitution of their immune system due to the administration of effective antiretroviral therapy [12]. In general, the severity of IRIS is directly related to the degree and rapidity of T cell recovery. Mouse models of PCP suggest that CD8+ T lymphocytes help modulate the inflammation produced by CD4+ T lymphocytes, but as mentioned above, their ineffectual inflammatory response can also contribute significantly to lung injury. These various T cell effects may be responsible for the variations in presentation and outcome of Pneumocystis pneumonia observed in different patient populations.
The inflammatory processes taking place during PCP do not appear to result in major long-term damage to the lung in those who recover. A long-term follow-up of 23 children with cancer and PCP showed a return to normal lung function by 6 months in all 18 survivors. Similar studies in adults are complicated by the fact that adult patients, especially those with AIDS, might have multiple pulmonary insults. While some studies, primarily of adult AIDS patients, suggest long-term pulmonary damage following PCP, other studies of renal transplant recipients have shown pulmonary function returned to nearly normal after recovery from PCP.
Clinical Manifestations
Pneumocystis Pneumonia
There are at least three distinct clinical presentations of PCP. In patients with profound immunodeficiency, such as young infants with congenital immunodeficiency, severe malnutrition, or in AIDS patients with very few CD4+ T lymphocytes, the onset of hypoxia and symptoms is subtle with cough, dyspnea on exertion, or tachypnea, often without fever. Infants may show progression to nasal flaring, intercostal, suprasternal, and infrasternal retractions. As the disease progresses patients develop hypoxia, with cyanosis in severe cases. In the sporadic form of PCP, occurring in children and adults with underlying immunodeficiency, the onset of hypoxia and symptoms is usually more abrupt with fever, tachypnea, dyspnea, and cough, progressing to severe respiratory compromise. This latter type accounts for the majority of cases, although the severity of clinical expression may vary. Rales are usually not detected on physical examination. The third pattern of disease is that associated with rapid restoration of immune function referred to as IRIS. It has been best described in newly diagnosed AIDS patients who are severely immunocompromised and present with PCP as their initial manifestation of AIDS [12]. These patients appear to respond well to therapy for PCP but 3–6 weeks after beginning treatment they experience an unexpected recurrence of pulmonary symptoms and chest X-ray (CXR) abnormalities that coincide with return of immune function. IRIS may also occur in bone marrow transplant patients who engraft while infected with Pneumocystis.
Extrapulmonary Infections
Extrapulmonary infection with Pneumocystis is rare. The incidence is not well defined, but is estimated to be a 1000-fold less likely than PCP itself [13]. The most commonly reported sites of infection include the ear and eye. Why these two sites seem to predominate is unclear but may reflect the fact that infection at these sites may quickly produce readily apparent signs and symptoms. Other sites of involvement are the thyroid gland, liver, kidney, bone marrow, lymph nodes, spleen, muscles, and gastrointestinal (GI) tract. How the organism arrives at these sites is unknown. Response to treatment is usually good when extrapulmonary infections occur in the absence of pulmonary infection.
Diagnosis
Pulmonary symptoms in at-risk patients should always raise the suspicion of PCP. The classic chest radiograph reveals bilateral diffuse alveolar disease with a granular pattern (see Fig. 6.7, Chap. 6). The earliest densities are perihilar, and progression proceeds peripherally, typically sparing the apical areas until last. Less common chest radiograph appearances in PCP include cystic lesions, pneumothorax, or isolated focal infiltrates. In patients receiving aerosolized pentamidine for prophylaxis, there may be a predisposition for upper lobe infiltrates. The arterial oxygen tension (PaO2) is invariably decreased.
A clinical pearl is that an elevated lactate dehydrogenase (LDH) may be a hint that one is dealing with PCP. This is due to the fact that LDH is a useful marker of alveolar and inflammatory cell damage. Because Pneumocystis is a diffuse alveolar infection, it tends to result in higher and more often elevated levels of LDH than some other more focal opportunistic pulmonary infections. For example, a recent analysis of LDH and pulmonary opportunistic infections in AIDS patients showed that about 90 % of those with definite PCP had elevated serum LDH [14]. Thus while not specific for PCP, very high LDH levels should raise one’s suspicion for PCP and normal levels make the diagnosis of PCP much less likely.
PCP can only be definitively diagnosed by demonstrating Pneumocystis in the lungs of a patient with compatible pulmonary signs and symptoms. Appropriate specimens for analysis include bronchoalveolar lavage, tracheal aspirate, transbronchial lung biopsy, bronchial brushings, percutaneous transthoracic needle aspiration, and open lung biopsy. Induced sputum samples are gaining popularity, but are helpful only if positive; the absence of Pneumocystis in an induced sputum sample does not exclude infection. The open lung biopsy is the most reliable method, although bronchoalveolar lavage is generally more practical. Estimates of the diagnostic yield of the various specimens are as follows: induced sputum 20–40 %, tracheal aspirate 50–60 %, bronchoalveolar lavage 75–95 %, transbronchial biopsy 75–95 %, and open lung biopsy 90–100 %. Once obtained, the specimens are typically stained with one of the four commonly used stains: Gomori methenamine silver (GMS) and toluidine blue stains only stain cyst forms; polychrome stains, such as Giemsa, stain both trophozoites and sporozoites; and the fluorescein-labeled monoclonal antibody also stains both trophozoites and cysts. Pneumocystis can also be visualized by Papanicolaou stain. Polymerase chain reaction analysis of respiratory specimens offers promise as a rapid diagnostic method, but a standardized system for clinical use has not been established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree